ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Effect of a cryopreservation protocol on the in vitro yield of adipose-derived stem cells
Influência de um protocolo de criopreservação no rendimento in vitro de células-tronco derivadas do tecido adiposo
Original Article -
Year2012 -
Volume27 -
Issue
3
Fernanda Ginani1; Diego Moura Soaresa1; Carlos Augusto Galvão Barboza2
ABSTRACT
BACKGROUND: Adipose tissue obtained by liposuction may be an accessible source of stem cells for future clinical application in tissue regeneration. Cryopreservation maintains stem cells in a live state for long periods of time, without impairing their function. The aim of this study was to assess the effect of a cryopreservation protocol on the proliferation of adipose-derived stem cells.
METHODS: Fragments of mouse adipose tissue were subjected to enzymatic digestion in order to isolate cells that were then cultured in minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS). Cells were maintained at 37ºC in 5% carbon dioxide (CO2). At the first passage, an aliquot of 1 × 106 cells was cryopreserved in FBS with 10% dimethylsulfoxide (DMSO) for 30 days, whereas the remaining cells were seeded and maintained in culture. When these cells reached the third passage, the 2 groups of cells (cryopreserved and non-cryopreserved) were seeded for experiments. Cell viability and proliferation curves were established at intervals of 24, 48, and 72 hours by counting cells with a hemocytometer and MTT assay. Nuclear morphology was assessed by DAPI staining. The data were statistically analyzed, with a significance level of 5%.
RESULTS: Increasing cellular proliferation was observed in both groups, with no significant difference throughout the experiment (P > 0.05). There was no significant change in cell viability and signs of nuclear damage were not detected in both the groups studied.
CONCLUSIONS: The cryopreservation protocol analyzed was effective for maintaining the integrity of adipose-derived stem cells, allowing their storage for later use.
Keywords:
Adipose tissue. Stem cells. Cryopreservation. Cell proliferation.
RESUMO
INTRODUÇÃO: O tecido adiposo obtido por lipoaspiração pode ser uma fonte acessível de células-tronco, para posterior aplicação clínica na regeneração tecidual. O processo de criopreservação mantém essas células vivas por longos períodos, sem prejuízo a suas funções. O objetivo deste estudo foi avaliar a influência de um protocolo de criopreservação na proliferação das células-tronco derivadas do tecido adiposo.
MÉTODO: Fragmentos de tecido adiposo de camundongos foram submetidos a digestão enzimática e as células foram cultivadas em meio α-MEM (do inglês Minimum Essential Medium), suplementado com 10% de soro fetal bovino (SFB), e mantidas a 37ºC em 5% de dióxido de carbono. No primeiro subcultivo, uma alíquota de 1x106 células foi criopreservada em SFB com 10% de dimetilsulfóxido por 30 dias, e outro grupo permaneceu em cultura. No terceiro subcultivo, as células dos dois grupos (não-criopreservadas e criopreservadas) foram plaqueadas e a viabilidade celular e as curvas de crescimento foram estabelecidas a partir de contagem em hemocitômetro e pelo ensaio de MTT, nos intervalos de 24 horas, 48 horas e 72 horas. A avaliação da morfologia nuclear foi realizada pela marcação por DAPI. Os dados foram analisados estatisticamente, com nível de significância de 5%.
RESULTADOS: Observou-se padrão de crescimento celular ascendente em ambos os grupos, sem diferença significante ao longo do experimento (P > 0,05). Não houve alteração considerável da viabilidade celular e danos nucleares também não foram observados nos grupos estudados.
CONCLUSÕES: O protocolo de criopreservação avaliado mostrou-se eficaz para manter a integridade das células-tronco de tecido adiposo, permitindo seu armazenamento para uso posterior.
Palavras-chave:
Tecido adiposo. Células-tronco. Criopreservação. Proliferação de células.
INTRODUCTION
Throughout life, adult stem cells are responsible for the replacement of cells in the tissues to which they belong. Adult stem cells have been isolated from several organs, including the highly complex adipose tissue1,2, which consists of mature adipocytes, pre-adipocytes, fibroblasts, endothelial cells, resident monocytes/macrophages, and lymphocytes3. Stem cell research has focused on the adipose tissue as a rich source of pluripotent stem cells4,5.
Stem cells isolated from adipose tissue (adipose tissuederived stem cells, ADSCs) have a capacity to differentiate that is similar to that of bone marrow-derived stem cells, thus leading eventually to mesodermal cells and tissues such as adipocytes, cartilage, bone, and skeletal muscle6. However, the main advantage of ADSCs is that they can be easily obtained from patients by a simple and minimally invasive procedure, in addition to being easily maintained in culture7.
The necessity to maintain live cells for a long time without compromising their functions led to the development of cryopreservation techniques. These procedures aim to inhibit, in a reversible and controlled manner, all of the biological functions of living tissues by storing cells at an ultra-low temperature that is usually around -196ºC8. Another possibility is to cryopreserve cells at a temperature of -80°C, which ensures similar results as those of preservation in liquid nitrogen, as reported by Woods et al.9. These authors cryopreserved undifferentiated mesenchymal cells of the dental pulp for 6 months at the 2 different temperatures mentioned above. They observed an identical capacity for cellular differentiation in both groups.
However, it is necessary to assess whether cryopreservation affects the proliferation of cryopreserved cells as well as their ability to differentiate. Problems with the cryopreservation process can hamper the restoration of biological functions of cells after thawing. Taking this into account, the aim of this study is to assess, with in vitro experiments, the effect of a simple cryopreservation protocol on the proliferative capacity of ADSCs.
METHODS
Isolation and Cell Culture
This study was approved by the Animal Research Ethics Committee (AREC) of the Federal University of Rio Grande do Norte, with the number 036/2012. Two Swiss albino mice, both males, aged 3 months, and weighing about 30 g, were euthanized with a lethal dose of sodium pentobarbital (150 mg/kg, intraperitoneal). Fragments of adipose tissue were isolated from the inguinal region. In a laminar flow tissue culture hood, the fragments were washed 3 times for 10 minutes each, with minimum essential medium (α-MEM; Cultilab, Brazil) supplemented with antibiotics and antifungal agents (Gibco, USA) to eliminate any possible contamination.
Subsequently, the adipose tissue was enzymatically digested with a solution containing 3 mg/mL collagenase I (Gibco, USA) and 1 mL trypsin/EDTA (Cultilab, Brazil) for 1 hour at 37ºC. Cells were then cultured in α-MEM supplemented with 10% fetal bovine serum (FBS) and maintained at 37ºC in 5% carbon dioxide (CO2) until reaching a confluence between 70% and 90%. The medium was changed every 3 days.
An aliquot of cells was cultured in osteogenic and adipogenic differentiation medium (StemPro® Differentiation Kits, Invitrogen Corp., USA) for 21 days. After this period, the cells presented the characteristic morphology of osteoblastic and adipose cells when observed under a light microscope. This confirmed the pluripotent phenotype of these cells.
Cryopreservation
At the first passage (P1), an aliquot of 1 × 106 cells was cryopreserved in FBS with 10% dimethylsulfoxide (DMSO), according to the following protocol: 2 hours at 4ºC, 18 hours at -20ºC, and then, storage at -80ºC for 30 days. After this period, cells were thawed and maintained in culture in the same way as cells that were not cryopreserved (P2).
Assessment of Proliferation and Cell Viability
At the third passage (P3), the 2 groups of cells (cryopreserved and non-cryopreserved) were seeded in quadruplicate (n = 4) at a density of 3 × 104 cells/well. Cellular yield and proliferation curves were assessed in the 2 groups at 24, 48, and 72 hours after seeding by counting cells with the trypan blue exclusion method. Cellular proliferation was also determined by [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) assay. For this experiment, cells were seeded in 96-well plates at a density of 5 × 103 cells/well. The time points mentioned above were also used for MTT assay. The optical density was obtained by reading the plates in a spectrophotometer at a wavelength of 570 nm.
Assessment of Morphological Nuclear Damage
Cell nuclei were stained with 4',6'-diamino-2-phenylindole (DAPI) in the 2 groups at 72 hours in order to identify possible apoptotic changes in nuclear morphology, such as chromatin condensation and nuclear fragmentation.
Statistical Analysis
The data were analyzed using nonparametric tests. The differences between the 2 groups for the time points considered (24, 48, and 72 hours) were assessed using the Mann-Whitney statistical test with a significance level of 5% (P < 0.05).
RESULTS
Increased proliferation was observed in both cryopreserved and non-cryopreserved cells. A higher proliferation rate was detected in cryopreserved cells (Figure 1).
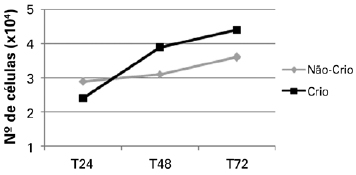
Figure 1 - Proliferation curves of non-cryopreserved and cryopreserved cells at the time points assessed. Cryo = cryopreserved; Non-Cryo = non-cryopreserved; T24 = 24 hours; T48 = 48 hours; T72 = 72 hours.
No statistically significant difference was observed by comparing the average trypan blue exclusion of cryopreserved and non-cryopreserved cells. There was also no significant difference in cell viability (Table 1) using this method. Similarly, no statistically significant difference in cellular proliferation was observed by MTT assay between the 2 groups at the time points considered (P > 0.05; Figure 2).
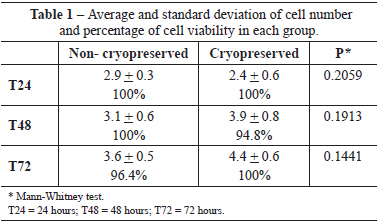
Figure 2 - Mitochondrial activity of adipose tissue-derived stem cells in the groups analyzed. Cryo = cryopreserved; Non-Cryo = non-cryopreserved; T24 = 24 hours; T48 = 48 hours; T72 = 72 hours.
In both groups, no morphological changes were observed in the nuclei of ADSCs (nuclear fragmentation or the presence of pyknotic nuclei; Figure 3).
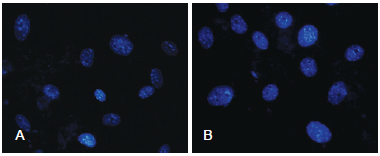
Figure 3 - In A, photomicrography of non-cryopreserved cells at 72 hours. In B, photomicrography of cryopreserved cells at 72 hours (DAPI, x40).
DISCUSSION
Nowadays, liposuction is a very common procedure performed in aesthetics clinics. With this surgery, a large amount of adipose tissue is easily obtained. In addition to being used as filling to increase the volume of soft tissues, this adipose tissue can also be used as a source of stem cells for tissue regeneration10. Therefore, cryopreservation has been investigated for the storage of adipose tissue (or specific cells) for later use.
Several studies have suggested different cryopreservation times, which vary from one day to longer periods11,12. The time used in the current article for our experiments corresponds to what we found in the literature, as several authors evaluated the cryopreservation effect after 30 days9,13,14 without observing significant differences in proliferation and differentiation capacity between cryopreserved cells and non-cryopreserved cells.
With the aim of assessing the pluripotent and proliferative capacity of human cryopreserved ADSCs, Gonda et al.12 stored these cells at low temperature for 6 months. After this period, they noticed that cryopreserved cells presented an osteogenic, adipogenic, and chondrogenic proliferation and differentiation capacity similar to that of non-cryopreserved cells. The difference between these groups that was highlighted by the authors was the higher variability in the chondrogenic differentiation capacity presented by cryopreserved cells. With regards to proliferation, these results are consistent with the results of our current study, as both groups (non-cryopreserved and cryopreserved cells) had an increasing cellular proliferation curve.
When assessing cryopreservation of ADSCs, Thirumala et al.15,16 mainly described the physical effect that the processes of freezing and thawing might have on the integrity of these cells. The results obtained by DAPI staining confirm that the cryopreservation protocol used in the present study conserved the nuclear membranes as well as the nuclear morphology of ADSCs.
Cryopreservation protocols for ADSCs suggest that these cells be stored at a temperature of -196ºC (liquid nitrogen) 12,17,18. This increases the cost of maintenance to levels that cannot be afforded by many research laboratories. In the current study, we used a simpler, more accessible, and low cost protocol by storing cells at a temperature of -80ºC, which allowed preservation of their viability.
Liu et al.19 observed results similar to those obtained in the present study when they cryopreserved human ADSCs for 2 weeks. In another study, dog ADSCs were cryopreserved for 12 months, and when thawed, they showed a proliferation rate similar to that of non-cryopreserved cells17. The time for which the cells remain cryopreserved may affect their viability. However, shorter periods, as used in this study (30 days), or longer periods (12 months), as assessed by Martinello et al.17, do not alter cellular proliferation. The data found in the literature suggest that the cryopreservation time for these cells can even be extended. However, the possible damage caused by longer periods of cryopreservation should be assessed in this specific cell type.
Among the factors that might influence cell cryopreservation, cryoprotectant agents may significantly impair the survival rate and proliferative capacity after thawing. In the current study, DMSO was chosen as a cryoprotectant, as it is commonly employed for the cryopreservation of different cell types11,17. As observed in our study, DMSO provided positive results in terms of cell viability and proliferative capacity after ADSC cryopreservation for a period of 30 days.
The rate with which the temperature decreases during freezing might be another important factor for cell viability after cryopreservation. Moreover, the appropriate rate of temperature decrease should be determined for each specific cell type in order to prevent the formation of intracellular ice crystals20. For other cells in culture, we adopted a standard protocol, as described by Vasconcelos et al.14 (2 hours at 4ºC, 18 hours at -20ºC, and, then, storage at -80ºC for 30 days), which also proved to be quite effective for ADSCs.
In general, the results of this study demonstrated that the cryopreservation protocol described might be an appropriate procedure for the storage of ADSCs, thus allowing the use of these cells in the investigation of future clinical applications in subsequent in vivo experiments.
CONCLUSIONS
The cryopreservation protocol tested was effective in maintaining the integrity of ADSCs, preserving their proliferation capacity without causing morphological nuclear damage.
REFERENCES
1. Levi B, Longaker MT. Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells. 2011;29(4):576-82.
2. De Rosa A, De Francesco F, Tirino V, Ferraro GA, Desiderio V, Paino F, et al. A new method for cryopreserving adipose-derived stem cells: an attractive and suitable large-scale and long-term cell banking technology. Tissue Eng Part C Methods. 2009;15(4):659-67.
3. Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, André M, et al. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579(17):3487-92.
4. Prunet-Marcassus B, Cousin B, Caton D, André M, Pénicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006;312(6):727-36.
5. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279-95.
6. Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13(2):77-81.
7. Sterodimas A, de Faria J, Nicaretta B, Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010;63(11):1886-92.
8. De Santis GC, Prata KL. Criopreservação de células-progenitoras hematopoéticas. Medicina. 2009;42(1):36-47.
9. Woods EJ, Perry BC, Hockema JJ, Larson L, Zhou D, Goebel WS. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 2009;59(2):150-7.
10. Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118(3 Suppl):121S-8.
11. Papaccio G, Graziano A, d'Aquino R, Graziano MF, Pirozzi G, Menditti D, et al. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208(2):319-25.
12. Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, Inoue K, et al. Preserved proliferative capacity and multipotency of human adiposederived stem cells after long-term cryopreservation. Plast Reconstr Surg. 2008;121(2):401-10.
13. Temmerman L, Beele H, Dermaut LR, Van Maele G, De Pauw GA.Influence of cryopreservation on the pulpal tissue of immature third molars in vitro. Cell Tissue Bank. 2010;11(3):281-9.
14. Vasconcelos RG, Ribeiro RA, Vasconcelos MG, Lima KC, Barboza CA. In vitro comparative analysis of cryopreservation of undifferentiated mesenchymal cells derived from human periodontal ligament. Cell Tissue Bank. 2011;13(3):461-9.
15. Thirumala S, Gimble JM, Devireddy RV. Transport phenomena during freezing of adipose tissue derived adult stem cells. Biotechnol Bioeng. 2005;92(3):372-83.
16. Thirumala S, Zvonic S, Floyd E, Gimble JM, Devireddy RV. Effect of various freezing parameters on the immediate post-thaw membrane integrity of adipose tissue derived adult stem cells. Biotechnol Prog. 2005;21(5):1511-24.
17. Martinello T, Bronzini I, Maccatrozzo L, Mollo A, Sampaolesi M, Mascarello F, et al. Canine adipose-derived-mesenchymal stem cells do not lose stem features after a long-term cryopreservation. Res Vet Sci. 2011;91(1):18-24.
18. Miyamoto Y, Oishi K, Yukawa H, Noguchi H, Sasaki M, Iwata H, et al. Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant. 2012;21(2-3): 617-22.
19. Liu G, Zhou H, Li Y, Li G, Cui L, Liu W, et al. Evaluation of the viability and osteogenic differentiation of cryopreserved human adipose-derived stem cells. Cryobiology. 2008;57(1):18-24.
20. Hubel A. Parameters of cell freezing: Implications for the cryopreservation of stem cells. Transfus Med Rev. 1997;11(3):224-33.
1. Specialist in Morphological Sciences, Masters Student of the Post-graduate Program in Public Health at the Universidade Federal do Rio Grande do Norte (UFRN), Natal, RN, Brazil.
2. Doctor in Oral Pathology, Associate Professor of the Department of Morphology at the UFRN, Natal, RN, Brazil.
Correspondence to:
Carlos Augusto Galvão Barboza
Departamento de Morfologia, Universidade Federal do Rio Grande do Norte
Av. Salgado Filho, 3.000 - Campus Universitário
Natal, RN, Brazil - CEP 59072-970
E-mail: cbarboza@cb.ufrn.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: June 12, 2012
Article accepted: August 7, 2012
Founder: CAPES
This study was performed at Universidade Federal do Rio Grande do Norte (UFRN), Natal, RN, Brazil.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license











