ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Recipient vessel options in microsurgical breast reconstruction
Escolha do vaso receptor em reconstrução de mama microcirúrgica
Original Article -
Year2013 -
Volume28 -
Issue
2
Maria Cecília Closs Ono1; Anne Karoline Groth2; Alfredo Benjamim Duarte da Silva3; Ivan Maluf Junior4
ABSTRACT
BACKGROUND: Microvascular transfer of autogenous tissue have become the gold standard for breast reconstruction. As in any free tissue reconstruction, recipient vessel choice is fundamental for adequate planning in breast reconstruction. The purpose of the present study is to determine which of the available recipient vessels (the internal mammary artery and its perforators vessels or circumflex scapular vessels) are adequate for microvascular breast reconstruction.
METHODS : A retrospective analysis of 117 consecutive patients who underwent microvascular breast reconstruction between January 2005 and December 2007 was performed. An algorithm that could be applied to the selection of the recipient vessel based in the axillary node dissection, immediate or late reconstruction, preoperative radiotherapy was established. Flap related complications, conversion rate and clinical outcomes were analised.
RESULTS: The internal mammary perforator, the internal mammary and the circumflex scapular are adequate recipient vessels for breast reconstruction, with similar rates of complications and viability. We also observed a lower flap viability rate when using superficial inferior epigastric artery flap comparing to deep inferior epigastric artery perfurator and transverse rectus abdominis musculocutaneous with muscle preservation flaps.
CONCLUSIONS: Microsurgical breast reconstruction is a safe and reliable method, with high flap viability and low complications.
Keywords:
Breast. Microsurgery. Surgical flaps.
RESUMO
INTRODUÇÃO: A transferência microvascular de tecido autógeno se tornou o padrão de referência para a reconstrução da mama. Como em qualquer reconstrução com tecido livre, a escolha do vaso receptor é fundamental para o planejamento adequado na reconstrução mamária. O objetivo do presente estudo é determinar quais dentre os vasos receptores disponíveis (a artéria mamária interna e seus vasos perfurantes ou os vasos circunflexos escapulares) são mais adequados para a reconstrução microvascular da mama.
MÉTODO: Foi realizada análise retrospectiva de 117 pacientes consecutivas submetidas a reconstrução da mama microvascular, entre janeiro de 2005 e dezembro de 2007. Foi estabelecido um algoritmo que pode ser aplicado para a seleção do vaso receptor com base em alguns parâmetros, como dissecção axilar, tempo da reconstrução (imediata ou tardia) e presença de radioterapia pré-operatória. Foram avaliadas as complicações relacionadas ao retalho, a taxa de conversão e os resultados clínicos.
RESULTADOS: A artéria mamária interna e seus vasos perfurantes e os vasos circunflexos escapulares são adequados para a reconstrução da mama, com taxas semelhantes de complicações e de viabilidade. Observou-se, também, maior risco de perda do retalho com o uso do retalho da artéria epigástrica inferior superficial em comparação ao retalho da artéria epigástrica inferior profunda ou retalho musculocutâneo abdominal transverso de músculo reto do abdome com preservação do músculo.
CONCLUSÕES: A reconstrução mamária microcirúrgica é um método seguro e confiável, com alta viabilidade do retalho e baixas taxas de complicação.
Palavras-chave:
Mama. Microcirurgia. Retalhos cirúrgicos.
INTRODUCTION
Microvascular breast reconstruction advantages are well known. The flap is better vascularised, allows better shaping, more natural result and less abdominal wall morbidity.
As in any free tissue reconstruction, recipient vessel choice is fundamental for adequate planning in breast reconstruction. The main concern is that any vessel diameter discrepancy may lead to thrombotic events, beside that, inadequate vessel choice affects the orientation and inset of the flap tissue, what may have significant impact on both the functional and aesthetic results1.
These concepts are of great importance when selecting recipient vessels for the free flap breast reconstruction.
Historically the thoracodorsal vessels were the preferred recipient vessels for breast reconstruction, but lately internal mammary vessels have gained popularity due to progressive reduction of axillary dissection routinely.
Many vessels can be used as recipient vessels in breast reconstruction, such as internal mammary, internal mammary perforator, thoracodorsal and scapular circumflex vessel; however each one holds individual characteristics that may present as advantages or disadvantages depending on the case.
The purpose of the present study is to determine whether the internal mammary and its perforators or circumflex scapular vessels are adequate recipient vessel for microvascular breast reconstruction, comparing flap related complications, conversion rates and clinical outcomes.
METHODS
A retrospective analysis of 117 consecutive patients who underwent microvascular breast reconstruction has been done between January 2005 and December 2007.
These patients were submitted whether to SIEA (superficial inferior epigastric artery), DIEP (deep inferior epigastric artery) or ms-TRAM (muscle sparing TRAM) flaps. Flap choice was determined after deep inferior epigastric perforator vessels assessment, if the perforator size was smaller than 1.5 mm the procedure adopted was ms-TRAM flap instead of DIEP flap.
Demographic data, risk factors for flap morbidity as smoking, preoperative radiation were assessed by the time of the reconstruction, the recipient vessels used. The clinical outcomes were collected from a chart review.
Outcomes including total flap loss, partial flap loss, vascular complications, fat necrosis and compromising of the mastectomy skin were determined. Vascular complications included the need of intraoperative anastomotic revision and postoperative reexploration of a flap for vascular insufficiency. Partial flap loss was defined as a partial flap ischemia resulting in loss of the skin paddle. Fat necrosis was defined as any clinically evident area of firmness in the breast that persisted beyond one year, whether or not it was radiologically or pathologically confirmed or required further intervention.
Figures 1 to 3 illustrate details of the dissection of the internal mammary artery and perforating vessels.
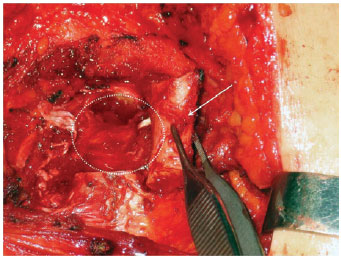
Figure 1 - Internal mammary vessels dissection. Costal cartilage ressection for dissection of the internal mammary vessels. White arrow: the costal cartilage being ressected. White dashed circle: region of probable identification of the internal mammary vessels.
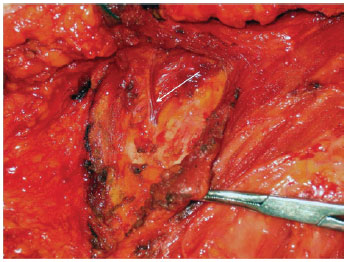
Figure 2 - Rectractors exposing the axilla. Internal mammary perforators dissection (white arrow).
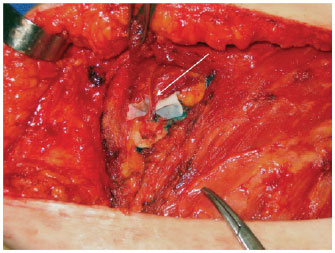
Figure 3 - Internal mammary perforators identification (white arrow).
All data were analyzed by descriptive statistics.
RESULTS
Data from 117 women were collected, 11 patients were submitted to bilateral reconstruction, totalizing 128 flaps.
The mean age was 46.1 ± 6.3, ranging from 23 to 72 years old. Sixty seven flaps were performed in the left side and 50 flaps in the right side.
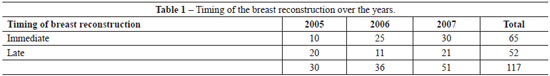
Sixty-five (55.6%) were submitted to immediate reconstruction and 52 (44.4%) to delayed reconstruction, and it was observed increase in immediate reconstruction over the years (Table 1).
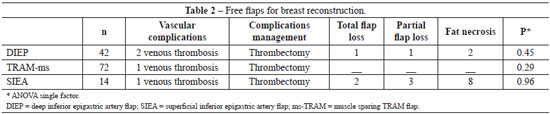
Forty two (32.8%) DIEP, 14 (10.9%) SIEA and 72 (56.3%) ms-TRAM flaps were performed. There were 5 vascular complications requiring reintervention, all of them due to venous thrombosis, and three evolved to total flap loss, 1 in DIEP group and 2 in the SIEA group.
Viability rate was 97.7% (125 flaps) in the series, but it was lower in the SIEA group. In the SIEA group there was a higher rate of fat necrosis (57.1%), which lead to a more specific indication of breast reconstruction using this flap. (Table 2)
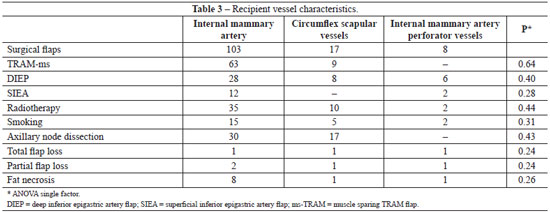
Regarding the recipient vessel we observed 99% of viability using internal mammary vessels, 94.1% with circumflex scapular and 87.5% with internal mammary perforator. Preoperative radiotherapy was administered in 40.2% of the patients, and those patients were preferably anastomosed to internal mammary vessels. Axillary node dissection was performed in 36.7% (Table 3).
It was necessary to convert from internal mammary vessel to circumflex scapular vessels in 11 cases, due to radiotherapy induced fibrosis. There was no need to convert from circumflex scapular or internal mammary perforator.
Regarding to the factors that influenced the selection of recipient vessels, if the patient had an immediate reconstruction and axillary node dissection at the time of reconstruction the circumflex scapular vessels were utilized preferably, in contrast, in delayed reconstruction or in the presence of preoperative radiotherapy internal mammary perforator or internal mammary vessels were preferred. In our series the thoracodorsal vessels were not utilized as recipient vessels for breast reconstruction.
DISCUSSION
Although the description of various different techniques for breast reconstruction, including those that uses silicone prosthesis2, autologous tissue transfer is considered to be the optimal method for breast reconstruction3-7.
The autologous methods for breast reconstruction are associated with higher rates of patient satisfaction and the reduction of undesirable results as capsular contracture and asymmetry2.
Since the first reports of microsurgical breast reconstruction8,9 the interest and the superiority of microvascular tissue transfer has been increasing10-12, specially because the more abundant blood flow, what enables the surgeon to transfer a greater amount of tissue, improving symmetry and decreasing the incidence of marginal ischemic complications13,14. Furthermore, little or no muscle is sacrificed bringing lower odds of donor-site morbidity15,16.
The selection of recipient vessels is of great importance for a successful microsurgical outcome, as the reliability and surgical accessibility of the donor vessel can affect flap survival, operative time and the orientation of the harvested flap (ipsilateral or contralateral)17.
For many years, the main choice of recipient vessels for microvascular autologous breast reconstruction following modified radical mastectomy was the thoracodorsal vessels. The blood flow rate in the recipient thoracodorsal artery has shown to be adequate, what has been proved by the utilization of an ultrasonic flowmeter in recipient vessels after the microsurgical anastomosis. The study showed that the intake of blood in TRAM flaps supplied by the internal mammary artery seems to be no greater than that in free flaps anastomosed to thoracodorsal vessels. As a conclusion, the blood supply in a free TRAM flap is independent of the flow in the recipient artery (as the flow in internal mammary was much higher) and it depends on the vascular bed of the flap, that reduces vascular resistance and equalizes the flow independently on the flow of the recipient vessel18. However, many surgeons has appointed disadvantages for their utilization as the difficult positioning during the procedure, the need of a long pedicle to reach the axillary region and the tendency for insetting of the flap to be shifted laterally in order to minimize tension on the vessels anastomoses1.
Moreover, with the advent of the sentinel node biopsy, thoracodorsal vessels had been no longer readily and systematically exposed making them a less convenient recipient vessels choice, especially with the possibility of a false-negative sentinel lymph node biopsy that may result in a posterior complementary axillary dissection17.
These difficulties have inspired the researches on another recipient vessels and the internal mammary system has started being used7,19-21. The internal mammary tends to remain protected from radiation fibrosis and it is not violated during a second breast ablative procedure20,22,23. Its dissection requires removal of costal cartilage. The criticism of sparing this vessel is that it might be necessary in cardiac revascularization procedures, therefore cardiovascular risk assessment is mandatory before surgery.
Lantieri et al.24 published a series of 40 free flaps for breast reconstruction using the circumflex scapular vessels as recipient vessels, to avoid the disadvantages related to internal mammary and thoracodorsal usage, and is presented as the author first choice. The vessels diameter ranged from 1.5 mm to 3 mm, and it was a branch of subscapular artery in 82.5% of the cases and a branch of axillary artery in the remaining 17.5%. Kawamura et al.25 in an anatomical study observed that the mean diameter of the circumflex scapular artery was 2.4 mm.
Recently Munhoz et al.26 published a series of internal mammary perforator as recipient vessels, advocating the costal cartilage and internal mammary vessels sparing27. However in our series we did not observe a high index of vessel compatibility, even in immediate reconstruction.
It is fundamental to choose the recipient vessel having in mind an alternative vessel in case of flap thrombosis and flap loss. In our series we have not used thoracodorsal vessels as recipient vessels to avoid losing the latissimus dorsi flap as a reconstructive option.
Based on all these arguments, we have established an algorithm that could be applied to the selection of the recipient vessel depending on immediate or late reconstruction, preoperative radiotherapy and axillary node dissection (Figure 4).
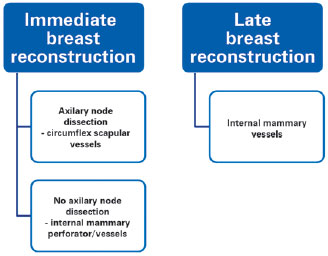
Figure 4 - Algorithm summarizing the choice of recipient vessels during microsurgical breast reconstruction.
CONCLUSIONS
The internal mammary perforator, the internal mammary and the circumflex scapular are adequate recipient vessels for breast reconstruction, with similar rates of complications and viability. We also observed a lower flap viability rate when using SIEA flap comparing to DIEP and ms-TRAM flaps.
The results demonstrate that it is possible to establish a protocol for choosing the recipient vessels for breast reconstruction, based in the axillary node dissection at the same time of breast reconstruction and radiotherapy as the two main factors to be analyzed for the best option.
Microsurgical breast reconstruction is a safe and reliable method, with high flap viability and low complications rates.
REFERENCES
1. Saint-Cyr M, Youssef A, Bae HW, Robb GL, Chang DW. Changing trends in recipient vessel selection for microvascular autologous breast reconstruction: an analysis of 1483 consecutive cases. Plast Reconstr Surg. 2007;119(7):1993-2000.
2. Park MC, Lee JH, Chung J, Lee SH. Use of internal mammary vessel perforator as a recipient vessel for free TRAM breast reconstruction. Ann Plast Surg. 2003;50(2):132-7.
3. Schusterman MA. The free TRAM flap. Clin Plast Surg. 1998;25(2):191-5.
4. Kroll SS, Baldwin B. A comparison of outcomes using three different methods of breast reconstruction. Plast Reconstr Surg. 1992;90(3):455-62.
5. McCraw JB, Horton CE, Grossman JA, Kaplan I, McMellin A. An early appraisal of the methods of tissue expansion and the transverse rectus abdominis musculocutaneous flap in reconstruction of the breast following mastectomy. Ann Plast Surg. 1987;18(2):93-113.
6. Nejedlý A, Tvrdek M, Kletenský J. Breast reconstruction using free tram flap transfer: ten years experience. Acta Chir Plast. 2001;43(2):35-8.
7. Schusterman MA, Kroll SS, Weldon ME. Immediate breast reconstruction: why the free TRAM over the conventional TRAM flap? Plast Reconstr Surg. 1992;90(2):255-61.
8. Fujino T, Harasina T, Aoyagi F. Reconstruction for aplasia of the breast and pectoral region by microvascular transfer of a free flap from the buttock. Plast Reconstr Surg. 1975;56(2):178-81.
9. Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg. 1979;13(3):423-27.
10. Elliott LF, Eskenazi L, Beegle PH Jr, Podres PE, Drazan L. Immediate TRAM flap breast reconstruction: 128 consecutive cases. Plast Reconstr Surg. 1993;92(2):217-27.
11. Kroll SS. Necrosis of abdominoplasty and other secondary flaps after TRAM flap breast reconstruction. Plast Reconstr Surg. 1994;94(5): 637-43.
12. Gherardini G, Arnander C, Gylbert L, Wickman M. Pedicled compared with free transverse rectus abdominis myocutaneous flaps in breast reconstruction. Scand J Plast Reconstr Surg Hand Surg. 1994;28(1):69-73.
13. Mehrara BJ, Santoro T, Smith A, Arcilla EA, Watson JP, Shaw WW, et al. Alternative venous outflow vessels in microvascular breast reconstruction. Plast Reconstr Surg. 2003;112(2):448-55.
14. Cunha MS, Munhoz AM, Sturtz G, Montag E, Ferreira MC. Evaluation of the inferior epigastric artery perforator flap perfusion in breast reconstruction. Rev Soc Bras Cir Plást. 2006;21(4):191-5.
15. Kroll SS, Schusterman MA, Reece GP, Miller MJ, Robb G, Evans G. Abdominal wall strength, bulging, and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg. 1995;96(3):616-9.
16. Nahabedian MY, Manson PN. Contour abnormalities of the abdomen after transverse rectus abdominis muscle flap breast reconstruction: a multifactorial analysis. Plast Reconstr Surg. 2002;109(1):81-7.
17. Moran SL, Nava G, Behnam AB, Serletti JM. An outcome analysis comparing the thoracodorsal and internal mammary vessels as recipient sites for microvascular breast reconstruction: a prospective study of 100 patients. Plast Reconstr Surg. 2003;111(6):1876-82.
18. Lorenzetti F, Kuokkanen H, von Smitten K, Asko-Seljavaara S. Intraoperative evaluation of blood flow in the internal mammary or thoracodorsal artery as a recipient vessel for a free TRAM flap. Ann Plast Surg. 2001;46(6):590-3.
19. Feng LJ. Recipient vessels in free-flap breast reconstruction: a study of the internal mammary and thoracodorsal vessels. Plast Reconstr Surg. 1997;99(2):405-16.
20. Dupin CL, Allen RJ, Glass CA, Bunch R. The internal mammary artery and vein as a recipient site for free-flap breast reconstruction: a report of 110 consecutive cases. Plast Reconstr Surg. 1996;98(4):685-9.
21. Clark CP 3rd, Rohrich RJ, Copit S, Pittman CE, Robinson J. An anatomic study of the internal mammary veins: clinical implications for free-tissue-transfer breast reconstruction. Plast Reconstr Surg. 1997;99(2):400-4.
22. Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg. 1999;52(2):104-11.
23. Evans GR, David CL, Loyer EM, Strom E, Waldron C, Ortega R, et al. The long-term effects of internal mammary chain irradiation and its role in the vascular supply of the pedicled transverse rectus abdominis musculocutaneous flap breast reconstruction. Ann Plast Surg. 1995;35(4):342-8.
24. Lantieri L, Serra M, Dallaserra M, Baruch J. Preservation of the muscle in the use of rectus abdominis free flap in breast reconstruction: from TRAM to DIEP (Deep inferior epigastric perforator) flap. Technical notes and results. Ann Chir Plast Esthet. 1997;42(2):156-9.
25. Kawamura K, Yajima H, Kobata Y, Shigematsu K, Takakura Y. Anatomy of Y-shaped configurations in the subscapular arterial system and clinical application to harvesting flow-through flaps. Plast Reconstr Surg. 2005;116(4):1082-9.
26. Munhoz AM, Ishida LH, Montag E, Sturtz GP, Saito FL, Rodrigues L, et al. Perforator flap breast reconstruction using internal mammary perforator branches as a recipient site: an anatomical and clinical analysis. Plast Reconstr Surg. 2004;114(1):62-8.
27. Munhoz AM. Internal mammary perforator recipient vessels for breast reconstruction using free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg. 2008;122(1):315-6.
1. Specialist member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), master in Surgical Clinics at the Universidade Federal do Paraná (Federal University of Paraná - UFPR), plastic surgeon at the Plastic Surgery Department of the Hospital Erasto Gaertner, Curitiba, PR, Brazil
2. Full member of the SBCP, master and PhD in Surgical Clinics at UFPR, plastic surgeon at the Plastic Surgery Department of the Hospital Erasto Gaertner, Curitiba, PR, Brazil
3. Full member of the SBCP, master and PhD in Plastic Surgery at the Universidade de São Paulo (University of são Paulo), plastic surgeon at the Plastic Surgery Department of the Hospital Erasto Gaertner, Curitiba, PR, Brazil
4. Resident physician at Reconstructive and Plastic Surgery Service of the Hospital de Clínicas of the UFPR, Curitiba, PR, Brazil
Correspondence to:
Maria Cecília Closs Ono
Rua Paulo Martins, 158 - casa 3 - Mercês
Curitiba, PR, Brazil - CEP 80710-010
E-mail: mccono@gmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: January 15, 2013
Article accepted: April 13, 2013
This study was performed at Hospital Erasto Gaertner, Curitiba, PR, Brazil.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license