ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Facial volume replacement with poly-l-lactic acid
Uso do ácido poli-L-láctico como restaurador de volume facial
Original Article -
Year2013 -
Volume28 -
Issue
2
Rosangela Maria Santini Ferreira da Silva1; Gustavo Félix Cardoso2
ABSTRACT
BACKGROUND: Poly-l-lactic acid has been used in Europe since 1999 for cosmetic purposes and in the United States since 2004 for the treatment of human immunodeficiency virus lipoatrophy. This paper aims to present the use of poly-l-lactic acid for the cosmetic purpose of restoring facial volume lost due to the aging process.
METHODS : Twelve patients were treated in private practice between 2006 and 2012. The product was reconstituted in 5 ml of distilled water and mixed with 2 ml of xylocaine 2% and epinephrine at the time of injection. Individualized treatment was performed according to each patient's facial size and contour. We analyzed photographs taken before and after surgery as well as subjective evaluations made by the physician and patients.
RESULTS: All patients were satisfied with the results. Adverse effects included 4 cases of ecchymosis at the injection site and 1 case of nodules in the periorbital region.
CONCLUSIONS: Poly-l-lactic acid is another tool for restoring, correcting, and smoothing facial deformities.
Keywords:
Cosmetic techniques. Face. Skin aging. Lactic acid.
RESUMO
INTRODUÇÃO: O ácido poli-L-láctico é usado na Europa, para fins cosméticos, desde 1999, e nos Estados Unidos, para tratamento de lipoatrofia pelo HIV, desde 2004. Este trabalho tem por objetivo apresentar nossa experiência com o emprego do ácido poli-L-láctico para uso cosmético, visando restaurar a perda de volume facial decorrente do processo de envelhecimento.
MÉTODO: Doze pacientes foram submetidos ao tratamento no período de 2006 a 2012. O produto foi reconstituído em 5 ml de água destilada e, no momento da injeção, foram acrescentados 2 ml de lidocaína a 2% com adrenalina 1:200.000. O tratamento foi individualizado de acordo com o volume e o contorno faciais dos pacientes. Os resultados foram analisados por meio de fotografias pré e pós-procedimento, e de acordo com a percepção do médico e dos pacientes.
RESULTADOS: Todos os pacientes mostraram-se satisfeitos com os resultados obtidos. Foram observados 4 casos de equimose no local da injeção, e 1 paciente apresentou nódulos na região periorbitária.
CONCLUSÕES: O ácido poli-L-láctico pode ser utilizado como mais uma ferramenta para restaurar, corrigir e amenizar as deformidades faciais.
Palavras-chave:
Técnicas cosméticas. Face. Envelhecimento da pele. Ácido láctico.
INTRODUCTION
The use of material injections in the face for esthetic improvement has been reported since 20081. With the improvement of anesthesia and surgical procedures during the second half of the 20th century, esthetic procedures have become more invasive. Fat tissue grafts were initially used for volume restoration after trauma. In the 20th century, autologous fat became the most used restoration material. However, removing and transporting fat is a lengthy and invasive process that does not lead to lasting effects in many cases2.
Poly-l-lactic acid was approved by the United States Food and Drug Administration (FDA) in August 2004 for the treatment of human immunodeficiency virus-induced lipodystrophy. The use of the technique for esthetic purposes has increased as well1,3. The use of poly-l-lactic acid is approved for cosmetic purposes in Europe, Canada, Australia, and Brazil.
Lactic acid polymers have been used for more than 30 years in different medical applications, such as re-absorbable suture threads, intra-bone grafts, and pins, plates, and screws for reconstructive surgery.
Poly-l-lactic acid is made of particles of poly-l-lactic acid, sodium carboxymethyl cellulose, and apyrogenic mannitol. It stimulates neocollagenesis, with results lasting up to 2 years, considerably longer than the time of degradation of poly-l-lactic acid in tissues (9 months), which occurs primarily through the respiratory excretion of carbon dioxide1.
The poly-l-lactic acid molecule, derived from corn dextrose fermentation, is heavy (140 kDa), crystalline, and 2-50 mm in diameter; non-enzymatic tissue hydrolysis leads to its degradation into lactic acid monomers. These monomers are phagocytized by macrophages and further degraded to glucose and carbon dioxide, which is eliminated through respiration. Although the mechanism by which poly-l-lactic acid restores tissue volume remains unknown, there is a common believe that this process is related to both the host response and gradual material degradation. Poly-l-lactic acid is biocompatible and biodegradable and carries no allergy testing requirement1.
Skin is the major indicator of age, health, and vitality. Solar exposure, acne, repetitive movements, and gravity lead to erosion and reflect an individual's age, while age-related physiological changes contribute to alterations in facial appearance4. Both intrinsic and extrinsic factors contribute to tissue aging throughout the body. Examples of extrinsic factors are cigarette smoke, solar exposure, alcohol consumption, and considerable weight gain and loss, among others.
With age, dynamic and static facial wrinkles become visible. Dynamic wrinkles result from muscle contraction. Static wrinkles, however, appear in the resting face due to loss of skin elastin, collagen, and hyaluronic acid caused by aging. Facial wrinkles are mostly due to loss of volume caused by lipoatrophy and altered fat distribution1.
Filling agents must be of non-animal origin, biocompatible, biodegradable, and non-permanent and have a low allergy risk and a low incidence of adverse effects such as edema, infection, migration, and tissue reactions1. Indications for their use include congenital abnormalities, scleroderma, Romberg syndrome, loss of facial fat tissue, and human immunodeficiency virus-mediated lipoatrophy. The use of injectable agents for both esthetic enhancement and repair is considered minimally invasive.
This study aims to present the authors' experience using poly-l-lactic acid for cosmetic purposes by restoring facial volume through the treatment of static wrinkles, particularly in the middle and lower thirds of the face.
METHODS
The data of 12 patients who were treated with poly-l-lactic acid between 2006 and 2012 were retrospectively studied. The average patient age was 58 (range, 43-73) years, and the group included 11 women and 1 man.
Facial volume and contour were examined, and particular focus was placed on the identification of concavities and convexities. In addition, overall tissue quality was assessed, as were other small alterations that could alter the activity of the injected agent.
Poly-l-lactic acid is a lyophilized product that requires reconstitution prior to use. Here, it was diluted in 5 ml of distilled water 24 hours before the procedure and mixed with 2 ml of lidocaine 2% and epinephrine 1:200,000 at the time of injection.
All patients underwent topical anesthesia with lidocaine 4% 20 minutes before the procedure. After the procedure, photos of the front, right, and left views of each patient were taken. The faces were then washed and disinfected with 70% alcohol with each patient in dorsal decubitus and slight torso inclination. Ice was used before the injection.
In the previously marked areas, deep dermal, subcutaneous, and supraperiosteal injections of 0.05-0.1 ml were performed using the tunneling technique (both centrifugal or efferent and centripetal or afferent directions) and serial puncture deposition with 26-30G needles.
Vigorous massage of the injected areas was performed immediately after the procedure and repeated for 30 days by the patients. The "5" rule was used because it complies with patient engagement; this rule includes 5 massages per day, each lasting 5 minutes, for 5 days after the procedure, followed by 1 massage per day for the next 25 days.
The injections were performed monthly in a total of 3 sessions, and 1 vial was used per session. The amount of poly-l-lactic acid used in each hemifacial injection was in agreement with the alterations observed in each patient. After 1.5-2 years, a new maintenance injection was performed.
The treated facial regions included the periorbital, superior zygomatic, nasolabial, malar, buccal, preauricular, and perioral commissures and the lower jaw line. Poly-l-lactic acid is not indicated for lip treatments.
Patients were photographed at the following times: before each poly-l-lactic acid injection; 4-6 weeks after the end of the treatment; and at 12 and 24 months after the end of the treatment. At each new session, the previous photographs were shown in the computer screen to allow for the monitoring of treatment progression. The results were evaluated by observing pre- and post-treatment photos.
Patient satisfaction was discussed at each session and the results were classified as "above expectations," "as expected," or "below expectations." Answers of "above expectations" and "as expected" were considered satisfactory. In addition, patient and doctor satisfaction concerning the appearance of the face were also analyzed. Faces that were elongated, concave, and with localized deformities became less elongated and without concavities or localized deformities after treatment.
RESULTS
All patients were satisfied with the results (Figure 1). Four cases of ecchymosis at the injection site were observed and 1 patient developed nodules in the periorbital region. These patients underwent incision and excision 4 months after the procedure (Figure 2).
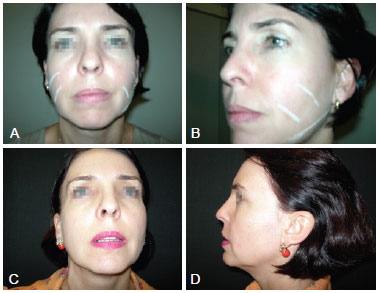
Figure 1 - A and B refer to pre-procedure photographs, with marks in the areas to be treated from the front and left side views, respectively. C and D refer to photographs taken 1 year post-procedure from the front and left side views, respectively.
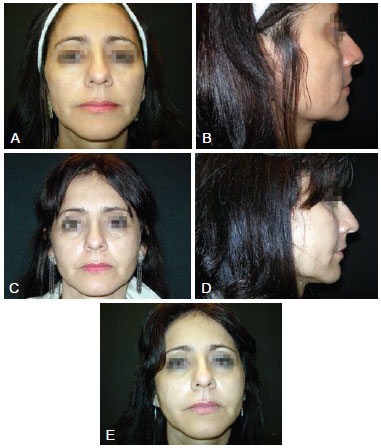
Figure 2 - A and B refer to photographs taken pre-procedure from the front and right side views, respectively. C refers to a photograph taken 4 months post-procedure, showing the presence of periorbital nodules in the right and left periorbital regions. D refers to a photograph taken 1 year post-procedure from right side view. E refers to a photograph taken 1 year post-procedure from the front view.
Medium and long-term assessments showed that all patients were satisfied with the methods and the results, including the patient who underwent nodule excision.
DISCUSSION
Age is associated with diminished facial volume due to several factors, including loss of collagen and elasticity, muscle flaccidness, lipoatrophy, alterations in fat tissue distribution, and bone reabsorption.
In the past, poly-l-lactic acid was reconstituted with 3 ml of distilled water 30 minutes before being injected. Nowadays, most clinicians reconstitute in 5 ml to 10 ml and add lidocaine 2%, after which the solution rests overnight and is agitated for 1 minute to become a homogeneous translucent gel. Vleegaar and Fritzgerald5 described supraperiosteal puncture sites for deep dermal or subdermal injections in the treatment of lipoatrophy areas and bone reabsorption areas.
Cosmetic use of poly-l-lactic acid for healthy individuals with photoaging is suitable not only in several facial areas (temples, periorbital region, nasogenian grooves, upper zygomatic areas, and malar, buccal, periauricular, and perioral commissures) but also in other areas of the body (hands, neck, acne scars, atrophic scars, and V-shaped deformities of the neck)1. Contraindications include hypersensitivity to poly-l-lactic acid or to sodium carboxymethyl cellulose, having previously submitted to filling with non-biodegradable agents, skin inflammation, or infection in the area to be treated.
Poly-l-lactic acid is not used for superficial wrinkle correction, nor is it used for radial lip line correction6-8.
Injection depth varies according to the area to be treated: deep dermal injections in the lower third of the face; deep dermal and subcutaneous injections in the medium third of the face; and sub-periosteal injections in the orbital contour by serial puncture with approximately 0.05 ml per puncture1.
The increase in collagen deposition follows polymer degradation in 6%, 32%, and 58% after 1, 3, and 6 months, respectively5.
Biopsy histologies performed at 8 and 24 months post-injection have shown that the progressive dissolution of poly-l-lactic acid is associated with an increase in type II collagen. Thirty months after the injection, microscopic observation showed the absence of poly-l-lactic acid and abundant collagen fibers.
Preliminary results are obtained after the first session, usually 30 days post-injection, with an average peak of activity around 6 months post-injection, lasting for about 2 years with the need for a maintenance session.
It is also possible to associate poly-l-lactic acid with botulinum toxin for the treatment of dynamic facial wrinkles and/or hyaluronic acid for the filling of accentuated furrows, creases, and rhytids. These products can be used simultaneously.
The most commonly observed adverse effects were hematomas, ecchymoses, and transient pain, which disappeared in a few days. Possible late complications are papules and nodules. In the majority of patients, papules and nodules disappear spontaneously 2-4 months after the injections. In the few cases when these adverse effects last, treatment is performed only after approximately 2 years, when the effects of the poly-l-lactic acid end3,8.
When the serial puncture technique is used in the superior zygomatic region, the needle must be introduced under the orbicularis oculi muscle and immediately above the periosteum. To reduce the incidence of nodules, it is important to distribute the agent uniformly by massaging the entire area after each injection.
The reconstitution of poly-l-lactic acid in 3 ml of distilled water must be specifically performed in patients with significant lipoatrophy, as these patients require a more intense response1. The study VEGA9 reported a high incidence of papules and nodules - approximately 52% - when dissolving poly-l-lactic acid in 3 ml of distilled water and following deep dermal injection. Nodule biopsies revealed reactions to foreign bodies.
Further diluting the poly-l-lactic acid agent with up to 11 ml of distilled water for use in the periorbital region has been recently reported.
In the 12 patients reported here, 4 cases of ecchymosis at the injection site were observed and 1 case of nodules in the periorbital region where the serial puncture had been performed was observed; these adverse effects were perceived 15 days post-procedure.
CONCLUSIONS
The esthetic results obtained with the use of poly-l-lactic acid are comparable to those observed with autologous fat grafts without the concerns and long durations associated with a surgical procedure and without the unpredictability that characterizes the behavior of fat tissues. Thus, poly-l-lactic acid is another tool for restoring, correcting, and smoothing facial deformities.
REFERENCES
1. Woerle B. Hanke CW, Sattler G. Poly-L-lactic acid: a temporary filler for soft tissue augmentation. J Drugs Dermatol. 2004;3(4):385-9.
2. Vleggaar D. Soft-tissue augmentation and the role of poly-L-lactic acid. Plast Reconstr Surg. 2006;118(3 Suppl):46S-54S.
3. Rotunda AM, Narins RS. Poly-L-lactic acid: a new dimension in soft tissue augmentation. Dermatol Ther. 2006;19(3):151-8.
4. Stewart DB, Morganroth GS, Mooney MA, Cohen J. Levin PS, Gladstone HB. Management of visible granulomas following periorbital injection of poly-L-lactic acid. Ophthal Plast Reconstr Surg. 2007;23(4):298-301.
5. Vleggaar D, Fitzgerald R. Dermatological implications of skeletal aging: a focus on supraperiosteal volumization for perioral rejuvenation. J Drugs Dermatol. 2008;7(3):209-20.
6. Dijkema SJ, van der lei B, Kibbelaar RE. New-fill injections may induce late-onset foreign body granulomatous reaction. Plast Reconstr Surg. 2005;115(5):76e-8e.
7. The Science of Dermal Fillers (Internet). Medscape CME Dermatology. Cited 2010 Aug 31. Disponível em: www.cme.medscape.com/dermatology.
8. Vleggaar D. Facial volumetric correction with injectable poly-L-lactic acid. Dermatol Surg. 2005;31(11 pt 2):1511-8.
9. Valantin MA, Aubron-Olivier C, Ghosn J, Laglenne E, Pauchard M, Schoen H, et al. Polylactic acid implants (new-fill) to correct facial lipoatrophy in HIV-infected patients: results of the open-label study VEGA. AIDS. 2003;17(17):2471-7.
1. Plastic surgeon, full member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), Brasília, DF, Brazil
2. Plastic surgeon, associated member of the SBCP, Brasília, DF, Brazil
Correspondence to:
Rosangela Maria Santini Ferreira da Silva
Av. L2 Sul - Quadra 607 - Sala 209 - Centro Clínico Metrópolis
Brasília, DF, Brazil - CEP 70200-670
E-mail: cliniser@globo.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: September 14, 2012
Article accepted: March 20, 2013
This study was performed at Clínica CliniSer, Brasília, DF, Brazil.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license









