ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Breast reconstruction with perforator flaps: use of the DIEAP flap at the Plastic Surgery and Microsurgery Service of Universidade Federal de Ciências da Saúde de Porto Alegre and Santa Casa de Misericórdia de Porto Alegre
Reconstrução da mama utilizando retalhos perfurantes: uso do DIEAP flap no Serviço de Cirurgia Plástica e Microcirurgia da Universidade Federal de Ciências da Saúde de Porto Alegre e da Irmandade Santa Casa de Misericórdia de Porto Alegre
ABSTRACT
INTRODUCTION: Great advances have been reported since the first abdominal tissue transfer carried out for breast reconstruction after a mastectomy. The deep inferior epigastric artery perforator (DIEAP) flap is one of the most recent advances in this area.
METHODS: In this article, we evaluate the important aspects in the field of autologous breast reconstruction with abdominal-based flaps, with emphasis on microsurgical flaps vascularized by perforating pedicles.
RESULTS: During the initial experience of this procedure, we were able to verify that the flap behaved according to what was reported in the literature.
CONCLUSION: The DIEAP flap provides a great degree of sculpting and volumetric gain to the reconstructed breast besides allowing a positive postoperative course for the patient.
Keywords:
Breast; Reconstruction; Perforator flaps.
RESUMO
INTRODUÇÃO: Uma grande evolução ocorreu desde o primeiro registro de transferência de tecido abdominal para reconstrução de mama pós-mastectomia. O retalho baseado em vasos perfurantes da artéria epigástrica inferior (DIEAP flap) apresenta-se com um dos mais recentes desenvolvimentos da área.
MÉTODOS: Este artigo analisa fatos importantes na área de reconstrução autóloga da mama utilizando retalhos baseados no abdome, com ênfase nos retalhos microcirúrgicos vascularizados por pedículos perfurantes.
RESULTADOS: Na experiência inicial do serviço, pudemos verificar que o retalho se comportou de acordo com a experiência relatada na literatura.
CONCLUSÃO: O DIEAP flap apresenta uma possibilidade maior de escultura e ganho volumétrico na mama reconstruída, além de evolução pós-operatória muito positiva.
Palavras-chave:
Mama; Reconstrução; Retalhos perfurantes.
INTRODUCTION
After a long period through which experience was gained and analysis necessary for the management of breast cancer was carried out, physicians have started to agree that reconstruction is an integral and inseparable part of the treatment. The quality of life, satisfaction with body image, and sexuality of mastectomized patients were reported to significantly improve after breast reconstruction1-3. The opportunity to be guided on reconstructive procedures should be offered4,5 to all patients submitted to mastectomy as a treatment option.
The procedures for breast reconstruction can usually be divided into the use of implants (tissue expanders and/or silicone implants) or autologous tissue (tissue removed from the same patient under treatment) to simulate the volume and shape of the breast. Several factors related to the characteristics of the patient and the disease influence the choice of the reconstruction technique5-7. Therefore, it is more and more important to reduce the morbidity associated with these procedures.
OBJECTIVES
The aim of this study is to review breast reconstructive procedures, with emphasis on abdominal-based autologous reconstructions and, in particular, on procedures involving the microsurgical transfer of flaps based on perforating vessels.
METHODS
This study was carried out in accordance with the guidelines for the research involving human beings recommended by the Declaration of Helsinki and the regulatory standards of the National Health Council for Human Research according to resolution CNS 196/96. The study was performed after obtaining the consent of the ethics committee of the institutions where it was conducted, namely the Irmandade Santa Casa de Misericórdia of Porto Alegre and the Federal University of Health Sciences of Porto Alegre.
Abdominal-based breast reconstructive procedures
In breast reconstruction, autologous tissues removed from different anatomical regions can be used to reconstruct the breast prominence. The abdomen provides a good quality and quantity of available tissue, along with the naturalness of the appearance of the resulting reconstruction. Moreover, this technique allows the improvement of body contour, as a secondary benefit resulting from the harmony of proportions between the thorax and the abdomen8.
Breast reconstruction - TRAM flap
Transverse rectus abdominis myocutaneous (TRAM) flap transfer was one of the first techniques introduced for autologous reconstruction that quickly became a treatment of choice in breast reconstruction8. The contribution of Hartrampf et al.9,10, in the beginning of the 1980s, to the establishment and improvement of this technique is worth mentioning. This flap is nourished by the perforating vessels connected to the rectus abdominis muscle and derives from deep epigastric vessels. After cutting the abdominal rectus muscle near its lower arcuate line, the deep inferior epigastric vessels are ligated. By means of subcutaneous tunneling, the abdominal tissues, superiorly pedicled to the rectus abdominis muscle, and the deep epigastric vessels are moved up to the breast region. The surgical steps crucial to reconstitute the anterior fascia of the rectus abdominis muscle and suture the other planes with the anatomical regions involved are then sequentially carried out. Volume adjustments, ipsilateral and contralateral breast shaping, and reconstruction of the nipple-papillary complex are usually postponed for subsequent procedures.
Breast reconstruction-microsurgical flaps
Vascular microsurgical techniques allow transposing distant tissues in only one surgery. Serafin et al. were the first to report the use of free flaps in breast reconstruction. Inguinal flaps were used for the correction of soft tissue defects of the thoracic wall11.
In 1979, Holmström was the pioneer in the use of abdominal tissue as a microsurgical flap for breast reconstruction - the free TRAM12. Notably, the procedure that Holmström described preceded publications on pedicle flaps. However, the limited availability and practicality of microsurgical techniques did not allow the fast acceptance of this procedure9,10.
Over time and with the widening use of microsurgery techniques, the advantages of a more stable perfusion through the deep inferior epigastric pedicle and the limitation of abdominal morbidity were recognized and therefore allowed considering TRAM microsurgical transfer as a reproducible and safe option for the reconstruction of the breast prominence13.
However, the use microsurgery to perform a complete tissue transfer potentially increases the incidence of total flap loss, which is higher when compared with the use of pedicled flaps, thus representing a factor to be considered in the choice of the technique to be used14.
Perforator vessel-based flaps
Studies have been carried out with the aim to reduce the morbidity of reconstructive procedures, which pertains in particular to the donor site, direct vessels, and vessels along the intramuscular and intracompartmental septa reaching the deep overlaying fascia, which in turn extends to the superficial fascia and the skin15. Koshima and Soeda16 were the first to report the use of flaps based on such vessels, known as perforating vessels.
Anatomical studies provided detailed descriptions of the skin vascular supply, reporting the presence of approximately 400 perforating vessels measuring >0.5 mm in diameter and located throughout the body. Therefore, any skin surface can be considered a potential donor area of perforator flaps.
Perforator vessel-based flaps allow the safe and reliable transfer of a patient's tissues and cause minimal donor site morbidity. The indications for the use of these flaps are similar to those of other autologous flaps.
Among the contraindications include a history of liposuction in the donor area and of active smoking, because the arterial supply of these flaps have an increased fragility compared with the pedicled flaps or the more robust free flaps such as free TRAM flaps17.
Breast reconstruction: perforator vessel- based vascularized flaps (abdomen)
Nowadays, the most commonly used flaps included in this category, especially for breast reconstruction, are the superficial inferior epigastric artery (SIEA) flap, or the muscle-sparing TRAM and DIEAP flaps.
The SIEA flap is based on the superficial inferior epigastric artery and exits intact through the fascia of the abdominal rectus muscle. Among the techniques described involving this muscle, this is the least invasive procedure. The vessels are smaller and the vascular pedicle is shorter when compared with the DIEAP. In several patients, the superficial vessels are absent or already cut during previous surgeries.
The muscle-sparing TRAM flap is an intermediate flap between the free TRAM and DIEAP flaps in terms of the spectrum of abdominal morbidity associated with the use of the lower abdomen as a donor area for breast reconstruction18. The procedure is similar to the use the free TRAM, although the amount of affected muscle is much lower, being approximately the size of a postage stamp. In a series of >400 cases19, this was the second technique most commonly used after the DIEAP flap. In fact, despite previous plans to perform DIEAP, the muscle-sparing procedure is eventually carried out, owing to the anatomical variability of the perforating vessels.
DIEAP flap
The DIEAP flap has been used for several reconstructive procedures. Allen and Treece20 were the first to carry out a successful breast reconstruction by transferring abdominal skin and adipose tissue in a procedure similar to the use of TRAM, saving the integrity of the abdominal muscle. While this technique provided the tissue required for the reconstruction, it also significantly reduced the morbidity of the abdominal wall21.
The DIEAP flap is based on the deep inferior epigastric artery and vein. The perforating vessels penetrate the rectus abdominis muscle on each side of the abdomen to provide the blood supply necessary for the adipose tissue and overlying skin. The deep inferior epigastric vessels have an outer diameter ranging between 2 and 3 mm. The perforating vessels are followed from the flap to their origins, in the inferior epigastric vessel, by means of a nontraumatic separation of the rectus abdominis fibers. During this dissection, the muscle and the fascia are spared.
The internal thoracic or dorsal thoracic vessels are the receiving vessels of choice. Usually, an end-to-end anastomosis is performed within the internal thoracic artery22.
The DIEAP flap is indicated for most patients that had been or will be submitted to mastectomy for the treatment of breast cancer and present a proper amount of tissue in the lower abdominal region. Congenital defects or defects occurring after conservative treatments and breast augmentation constitute other possible indications for this procedure. The contraindications are the same for all perforator vessel-based flaps17. Although considered not the best indication for this technique18,23, several reports recommend this procedure for the treatment of upper and/or lower abdominal scars.
The preoperative evaluation of blood vessels through Doppler ultrasonography is of great utility24. The common markings of abdominoplasty are carried out with the patients in the orthostatic or sitting position. In general, one team prepares the receiving vessels, while another elevates the flap18.
When DIEAP is performed, after the incisions, the first vessels to be approached are the inferior epigastric superficial vessels, which, for the purpose of providing suitable size and quality, allows carrying out a SIEA flap transfer or the use of a SIEA flap as an anastomotic back-up25.
The abdominal skin island is carefully elevated from the lateral to the medial side, paying special attention when the lateral edge of the rectus abdominis starts to appear. If the lateral side is not providing perforating vessels of good caliber, these are obtained from the medial portion. If no perforating vessels seem to be adequate in terms of size and quality, two or more small perforating vessels can be used in the procedure. Most of the perforator flaps are based on two vessels15. Although the experience of the surgeon helps in deciding which perforating vessel would need to be used, it is common to choose the larger identified vessel as soon as it exits through the anterior sheath of the rectus abdominis muscle. It is common to find these vessels close to the umbilical scar. After identifying the perforating vessel, the sheath of the rectus abdominis is carefully dissected and the vascular course is followed in the muscle.
Once the perforating vessels are chosen, these are dissected through a small opening in the rectus sheath. The intercostal nerves, innervating the medial aspect of the muscle, should be preserved. The dissection continues until the pedicle presents an adequate length, between 8 and 10 cm.
Under optical magnification, the procedure continues with a venous and arterial anastomosis, carried out by using a typical 9-0 nylon thread. Then, the microvascular clamp is released while verifying capillary perfusion and checking for bleeding of the flap.
The abdominal fascia is then sutured, with no need for meshes or other synthetic materials. The subsequent suture is carried out as in abdominoplasty procedures.
During the positioning of the flap for the reconstruction, the region between the extremity of the contralateral side and the pedicle (area 4) is usually removed26.
After the procedure, the postoperative period is preferably carried out in the intensive care unit or recovery room (intermediate care), where the patient remains for the first 24 h. Postoperative pain is significantly lower than that from procedures that do not spare the abdominal muscle27. Walking is encouraged 24 h after the surgery, and hospital discharge usually starts 3 days after.
RESULTS
The morbidity profile presented by the DIEAP flap is favorable when compared with that of other techniques of breast autologous reconstruction.
Fat necrosis seems to be the most frequent complication. Partial flap loss seems to account for 2.5% of the cases in the different cohorts evaluated, and the total loss rate reported is not higher than 1%17-19,28. The incidence of other complications, e.g., infection, hematoma, and epidermolysis, is similar to that of other procedures of autologous reconstruction.
The morbidity of abdominal DIEAP presents a lower incidence concerning hernias and concavities of the abdominal wall in comparison with TRAM27.
However, there is a trade-off to be considered when TRAM, muscle-sparing TRAM, DIEAP, and SIEA flaps are used for breast reconstruction; that is, the smaller the damage caused to the muscle and the fascia in the attempt to minimize abdominal morbidity, the fewer the included perforating vessels and the higher the risk of complications, e.g., fat necrosis and flap loss29,30.
DISCUSSION
The first experience of the authors in the production of the DIEAP flap used for breast reconstruction was very positive, and the flap behaved as reported in the literature (Figures 1 to 3).
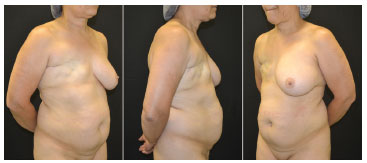
Figure 1. Preoperative pictures. Female patient, 51 years old, submitted to modified radical mastectomy and axillary clearance for invasive ductal carcinoma.
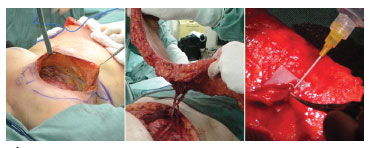
Figure 2. Transoperative pictures. Left: receiving area. Center: dissected flap. Right: end-terminal vascular anastomosis between the deep inferior epigastric vessels and the ipsilateral internal mammary vessels, and the anatomical mastectomy defect.
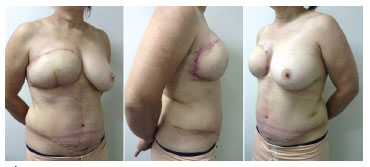
Figure 3. Pictures taken 21 days after the DIEAP flap surgical procedure. Left: left profile view. Center: right profile view. Right: profile view. Mammoplasty for breast symmetrization, reproduction of the nipple-areola complex with a tattoo, and skate flap placement will be performed in subsequent surgeries.
After the implementation of this surgery, we believe that, despite the increased surgical time, it presents greater possibilities of breast sculpting and volumetric gain of the flap, in addition to the positive course of the patient during the hospitalization. It should be clarified to the patient that complete flap loss is possible. The possibility that the abdominal contour may improve in a sensitive manner, without major damage to the muscles, and that the loss of the flap would not permanently prevent the patient from undergoing other reconstructive procedures represent the potential benefits of this technique.
CONCLUSION
The use of flaps based on perforating vessels may reverse the tendency to indicate implant-based reconstructions to patients at risk. The implementation of flaps vascularized by perforating pedicles in daily clinical-surgery practice may represent a fair choice for patients and further improve the important field of plastic surgery.
ACKNOWLEDGMENTS
We thank the residents and graduating students of the Plastic Surgery and Microsurgery Service of UFCSPA-ISCMPA who contributed to the establishment of this microsurgery training system and donated their time to this study.
REFERENCES
1. Trejo-Ochoa JL, Maffuz-Aziz A, Said-Lemus FM, Dominguez-Reyes CA, Hernández-Hernández B, Villegas-Carlos F, et al. [Impact on quality of life with breast reconstructive surgery after mastectomy for breast cancer]. Ginecol Obstet Mex. 2013;81(9):510-8. PMid:24187814.
2. Gopie JP, ter Kuile MM, Timman R, Mureau MA, Tibben A. Impact of delayed implant and DIEP flap breast reconstruction on body image and sexual satisfaction: a prospective follow-up study. Psychooncology. 2014;23(1):100-7. http://dx.doi.org/10.1002/pon.3377. PMid:23983109
3. Hernandez-Boussard T, Zeidler K, Barzin A, Lee G, Curtin C. Breast reconstruction national trends and healthcare implications. Breast J. 2013;19(5):463-9. PMid:23758582.
4. Association of Breast Surgery at Baso 2009. Surgical guidelines for the management of breast cancer. Eur J Surg Oncol. 2009;35(Suppl 1):1-22. http://dx.doi.org/10.1016/j.ejso.2009.01.008. PMid:19299100
5. Sociedade Brasileira de Mastologia, Sociedade Brasileira de Cancerologia. Diretrizes da Sociedade Brasileira de Mastologia e Sociedade Brasileira de Cancerologia no Tratamento Cirúrgico do Câncer de Mama. São Paulo: Associação Médica Brasileira e Conselho Federal de Medicina; 2011.
6. Grover R, Padula WV, Van Vliet M, Ridgway EB. Comparing five alternative methods of breast reconstruction surgery: a cost-effectiveness analysis. Plast Reconstr Surg. 2013;132(5):709e-23e. http://dx.doi.org/10.1097/PRS.0b013e3182a48b10. PMid:24165623
7. Zhong T, Hu J, Bagher S, O'Neill AC, Beber B, Hofer SO, et al. Decision regret following breast reconstruction: the role of self-efficacy and satisfaction with information in the preoperative period. Plast Reconstr Surg. 2013;132(5):724e-34e. http://dx.doi.org/10.1097/PRS.0b013e3182a3bf5d.PMid:24165624
8. Elliott LF, Hartrampf CR Jr. Breast reconstruction: progress in the past decade. World J Surg. 1990;14(6):763-75. http://dx.doi.org/10.1007/BF01670523. PMid:2256348
9. Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69(2):216-25. http://dx.doi.org/10.1097/00006534-198202000-00006. PMid:6459602
10. Hartrampf CR Jr, Bennett GK. Autogenous tissue reconstruction in the mastectomy patient. A critical review of 300 patients. Ann Surg. 1987;205(5):508-19. http://dx.doi.org/10.1097/00000658-198705000-00009. PMid:2953315
11. Serafin D, Georgiade NG, Given KS. Transfer of free flaps to provide well-vascularized, thick cover for breast reconstructions after radical mastectomy. Plast Reconstr Surg. 1978;62(4):527-36. http://dx.doi.org/10.1097/00006534-197810000-00005. PMid:358232
12. Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg. 1979;13(3):423-7. http://dx.doi.org/10.3109/02844317909013092. PMid:396670
13. Grotting JC, Urist MM, Maddox WA, Vasconez LO. Conventional TRAM flap versus free microsurgical TRAM flap for immediate breast reconstruction. Plast Reconstr Surg. 1989;83(5):828-41, discussion 842-4. http://dx.doi.org/10.1097/00006534-198905000-00009. PMid:2523544
14. Namnoum JD. Pedicle versus free TRAM for breast reconstruction. Breast Dis. 2002;16:79-83. PMid:15687660.
15. Khan FN, Spiegel AJ. The evolution of perforator flaps. Semin Plast Surg. 2006;20(2):53-5. http://dx.doi.org/10.1055/s-2006-941710.
16. Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42(6):645-8. http://dx.doi.org/10.1016/0007-1226(89)90075-1. PMid:2605399
17. Granzow JW, Levine JL, Chiu ES, Allen RJ. Breast reconstruction using perforator flaps. J Surg Oncol. 2006;94(6):441-54. http://dx.doi.org/10.1002/jso.20481. PMid:17061279
18. Chang DW. Breast reconstruction with microvascular MSTRAM and DIEP flaps. Arch Plast Surg. 2012;39(1):3-10. http://dx.doi.org/10.5999/aps.2012.39.1.3. PMid:22783484
19. Damen TH, Morritt AN, Zhong T, Ahmad J, Hofer SO. Improving outcomes in microsurgical breast reconstruction: lessons learnt from 406 consecutive DIEP/TRAM flaps performed by a single surgeon. J Plast Reconstr Aesthet Surg. 2013;66(8):1032-8. http://dx.doi.org/10.1016/j.bjps.2013.04.021. PMid:23642795
20. Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32(1):32-8. http://dx.doi.org/10.1097/00000637-199401000-00007. PMid:8141534
21. Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg. 2009;124(3):752-64. http://dx.doi.org/10.1097/PRS.0b013e31818b7533. PMid:19342994
22. Ono MCC, Groth AK, Silva ABD, Maluf I Jr. Escolha do vaso receptor em reconstrução de mama microcirúrgica. Rev Bras Cir Plást. 2013;28(2):227-32. http://dx.doi.org/10.1590/S1983-51752013000200010.
23. Hamdi M, Larsen M, Craggs B, Vanmierlo B, Zeltzer A. Harvesting free abdominal perforator flaps in the presence of previous upper abdominal scars. J Plast Reconstr Aesthet Surg. 2014;67(2):219-25. http://dx.doi.org/10.1016/j.bjps.2013.10.047. PMid:24280540
24. Pratt GF, Rozen WM, Chubb D, Ashton MW, Alonso-Burgos A, Whitaker IS. Preoperative imaging for perforator flaps in reconstructive surgery: a systematic review of the evidence for current techniques. Ann Plast Surg. 2012;69(1):3-9. http://dx.doi.org/10.1097/SPA.0b013e318222b7b7. PMid:22627495
25. Sbitany H, Mirzabeigi MN, Kovach SJ, Wu LC, Serletti JM. Strategies for recognizing and managing intraoperative venous congestion in abdominally based autologous breast reconstruction. Plast Reconstr Surg. 2012;129(4):809-15. http://dx.doi.org/10.1097/PRS.0b013e318244222d. PMid:22456352
26. Cunha MS, Munhoz AM, Sturtz G, Montag E, Ferreira MC. Avaliação de perfusão do retalho perfurante da artéria epigástrica inferior microcirúrgico aplicado em reconstrução mamária. Rev Bras Cir Plást. 2006;21(4):191-5.
27. Chang EI, Chang EI, Soto-Miranda MA, Zhang H, Nosrati N, Robb GL, et al. Comprehensive analysis of donor-site morbidity in abdominally based free flap breast reconstruction. Plast Reconstr Surg. 2013;132(6):1383-91. PMid:24005365.
28. Sinna R, Boloorchi A, Mahajan AL, Qassemyar Q, Robbe M. What should define a "perforator flap"? Plast Reconstr Surg. 2010;126(6):2258-63. http://dx.doi.org/10.1097/PRS.0b013e3181f61824. PMid:21124168
29. Atisha D, Alderman AK. A systematic review of abdominal wall function following abdominal flaps for postmastectomy breast reconstruction. Ann Plast Surg. 2009;63(2):222-30. http://dx.doi.org/10.1097/SAP.0b013e31818c4a9e. PMid:19593108
30. Rozen WM, Whitaker IS, Chubb D, Ashton MW. Perforator number predicts fat necrosis in a prospective analysis of breast reconstruction with free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg. 2010;126(6):2286-8, author reply 2288-9. http://dx.doi.org/10.1097/PRS.0b013e3181f61c04. PMid:21124185
1. Adjuct Professor of Plastic Surgery at the Federal University of Health Sciences of Porto Alegre (UFCSPA), Preceptor of the Plastic Surgery and Microsurgery Service at the Federal University of Heath Sciences of Porto Alegre (UFCSPA/ISCMPA), Porto Alegre, RS, Brazil
2. Member of the Brazilian Society of Plastic Surgery (BSPS), Resident at the Plastic Surgery and Microsurgery Service of the Federal University of Heath Sciences of Porto Alegre (UFCSPA/ISCMPA), Porto Alegre, RS, Brazil
3. Titular Member of the Brazilian Society of Plastic Surgery (BSPS), Preceptor of the Plastic Surgery and Microsurgery Service at the Federal University of Heath Sciences of Porto Alegre (UFCSPA/ISCMPA), Porto Alegre, RS, Brazil
4. Member of the Brazilian Society of Plastic Surgery (BSPS), Preceptor of the Plastic Surgery and Microsurgery Service at the Federal University of Heath Sciences of Porto Alegre (UFCSPA/ISCMPA), Porto Alegre, RS, Brazil
5. Medical Student at the Federal University of Heath Sciences of Porto Alegre (UFCSPA), Porto Alegre, RS, Brazil
6. Chief of the Plastic Surgery and Microsurgery Service at the Federal University of Health Sciences of Porto Alegre (UFCSPA/ISCMPA), Adjunct Professor of Plastic Surgery at the Federal University of Heath Sciences of Porto Alegre (UFCSPA), Porto Alegre, RS, Brazil
Institution: Work performed at the Plastic Surgery and Microsurgery Service of the Federal University of Health Sciences of Porto Alegre (UFCSPA) and at the Irmandade Santa Casa de Misericórdia of Porto Alegre (ISCMPA), Porto Alegre, RS, Brazil.
Corresponding author:
Barbara D'Avila Goldoni
Rua Alfredo Petry, 49 - Novo Esteio
Esteio, RS, Brazil ZIP 93270-580
Phone: +55 (51) 8408-3992
E-mail: bdgoldoni@gmail.com
Article received: January 5, 2014.
Article accepted: August 3, 2014.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license










