

Original Article - Year 2012 - Volume 27 -
Hemostatic net: an alternative for the prevention of hematoma in rhytidoplasty
Rede hemostática: uma alternativa para a prevenção de hematoma em ritidoplastia
ABSTRACT
BACKGROUND: Hematoma is the most common postoperative complication of rhytidoplasty. Its occurrence increases morbidity and impairs recovery. Internal sutures to close detached areas are used in abdominoplasty to prevent seromas. Taking this into account and in order to reduce the number of patients with hematomas post rhytidoplasty, we have developed a similar surgical procedure in which a hemostatic net is made of continuous nylon 4-0 transfixing stitches to include the skin and the superficial musculoaponeurotic system-platysma.
METHODS: A total of 366 patients who underwent rhytidoplasty of at least the middle third of the face between July 2009 and September 2011 were included in the study. Group A was identified as the control group; it included the first 120 patients assessed retrospectively. The remaining 246 patients, who underwent the surgical procedure here proposed and who were evaluated prospectively formed group B. We observed the incidence of hematoma, ischemia, and necrosis in the first 72 postoperative hours.
RESULTS: In group A, 17 (14.2%) patients developed hematomas, whereas none occurred in group B. The surgical procedure did not significantly increase the occurrence of ischemia: 11 (9.2%) patients in group A and 16 (6.5%) in group B had this complication (P < 0.3964). There was also no significant increase in the incidence of necrosis, which was observed in 3 (2.5%) patients in group A and 4 (1.6%) in group B (P < 0.4298).
CONCLUSIONS: The hemostatic net is an efficient method for the prevention of early hematomas following rhytidoplasty. This surgical procedure did not result in a significant increase in the incidence of ischemia and necrosis.
Keywords: Hematoma. Rhytidoplasty. Face. Necrosis. Ischemia.
RESUMO
INTRODUÇÃO: Hematoma é a complicação pós-operatória mais frequente em ritidoplastia. Sua ocorrência aumenta a morbidade e prejudica a recuperação. Pontos de adesão internos para fechamento das áreas descoladas em abdominoplastias evitam o surgimento de seroma. Baseados neste princípio, e com o objetivo de reduzir o número de pacientes com hematomas em ritidoplastia, foi desenvolvida tática cirúrgica análoga em que se confecciona uma rede hemostática de pontos contínuos e transfixantes de náilon 4-0, englobando a pele e o SMAS-platisma.
MÉTODO: Foram incluídos no estudo 366 pacientes consecutivos, submetidos a ritidoplastia de pelo menos o terço médio da face, entre julho de 2009 e setembro de 2011. O grupo A, incluindo os primeiros 120 pacientes avaliados retrospectivamente, foi considerado controle. O grupo B foi constituído pelos demais 246 pacientes, operados com a tática cirúrgica proposta e avaliados prospectivamente. Observaram-se as incidências de hematoma, isquemia e necrose nas primeiras 72 horas de pós-operatório.
RESULTADOS: No grupo A, 17 (14,2%) pacientes apresentaram hematoma, enquanto no grupo B não houve nenhum caso. A tática cirúrgica não aumentou significativamente a ocorrência de isquemia: 11 (9,2%) pacientes no grupo A e 16 (6,5%) no grupo B tiveram essa complicação (P < 0,3964). Também não houve alteração significativa na incidência de necrose, complicação observada em 3 (2,5%) pacientes do grupo A e em 4 (1,6%) do grupo B (P < 0,4298).
CONCLUSÕES: A rede hemostática é método eficaz na prevenção de hematomas precoces em ritidoplastias. Essa tática cirúrgica não levou a aumento significativo da incidência de isquemia e necrose.
Palavras-chave: Hematoma. Ritidoplastia. Face. Necrose. Isquemia.
Hematoma is the most frequent postoperative complication of rhytidoplasty1-3, occurring in up to 8% of patients4. Its occurrence affects postoperative recovery, as it is associated with an increased incidence of edema, ecchymosis, and, in some cases, ischemia, infection, and necrosis of the operated area1.
Over the past 4 decades, plastic surgeons have been looking for a solution to this problem without, however, finding a definitive answer to it. Neither drains5,6, tissue glues7-10, tumescent infiltration11, nor dissection with ultrasonic instruments12 has been completely effective in preventing hematoma. On the other hand, studies that have observed no incidence of this complication have been based on the control of clinical variables that are sometimes difficult to reproduce13.
Seroma is a common complication after other plastic surgery procedures, especially abdominoplasty; however, there is an efficient and technically feasible solution to the problem. As conceived by Baroudi and Ferreira14, it consists of an internal suture that leads to almost total closure of the detached area, thus avoiding eventual fluid deposits. In rhytidoplasty, the use of internal sutures is complicated due to the dynamic traction of the flaps and its inadequate accommodation under the skin. In these operations, the use of external sutures to stabilize the flap has already been described by Pontes15.
Based on the principle of mechanical and compulsory closure of the operated areas, as occurs in abdominoplasty, we describe here a surgical procedure in which a homeostatic net of continuous and transfixing sutures in the skin is created. This leads to almost total occlusion of the operated area.
METHODS
From July 2009 to September 2011, 366 patients with indications for rhytidoplasty were operated upon by the same surgical team in the same hospital. All patients were photographed during the preoperative and postoperative periods.
All patients on whom we performed rhytidoplasty of at least the middle third of the face, whether or not it was associated with blepharoplasty, endoscopic treatment of the frontal region, and/or platysmaplasty, were included in the study. No patient with these characteristics was excluded during the study period.
The patients were divided in two groups. Group A included the first 120 patients studied, who were operated on between July 2009 and March 2010. These patients were considered the control group and were not submitted to the proposed surgical procedure. Retrospective data were collected from this group. Group B was composed of the remaining 246 patients, upon whom the surgery was performed between April 2010 and September 2011, using the proposed surgical procedure. Data were collected prospectively from this group.
We collected descriptive data regarding age, gender, number of previous rhytidoplasties, association with submental platysmaplasty, upper and lower blepharoplasty, frontal sinus endoscopy, rhinoplasty, body-contouring surgery (breast reduction or augmentation, abdominoplasty, and liposuction), smoking, and hypertension.
All patients were observed for the occurrence of hematoma, ischemia, and necrosis in the first 3 postoperative days. Hematoma, as previously defined4,16, is a blood deposit of over 30 mL that requires surgical drainage in the operating room. Ischemia was defined clinically as an area of 1 cm2 (or more) of purplish color, where the capillary refill test, measured by digital compression and needle puncture, was slower than that in the adjacent area with normal skin color. Necrosis was defined as a blackened area of 1 cm2 (or more), in which there was no perfusion, according to the criteria described above.
All patients agreed to sign an informed consent form. Patients of group B (and their relatives, whenever possible) received guidance about the proposed surgical procedure before surgery, with the help of videos and photos.
This work was approved by the Research Ethics Committee of the Beneficent Evangelical Society of Curitiba on July 21, 2009.
Surgical procedures and strategy proposed
All patients were anesthetized by the same group of 5 anesthesiologists (the designation was made randomly, based on the anesthesiology table of weekly service). The anesthesia was applied under local sedation.
A combination of midazolam, ketamine, propofol, and fentanyl was used for sedation17. Clonidine was also used in most patients during the induction of anesthesia, for better blood pressure control18. Cephalexin (1 g) and dexamethasone (10 mg) intravenous infusions were given during induction of anesthesia.
After induction, the patients were infiltrated with two tumescent local anesthetic solutions16. The first, for use at the incisions was more concentrated and was composed of 20 mL of lidocaine 2%, 10 mL of ropivacaine 1%, and 220 mL of saline solution 0.9%, and epinephrine 1:400,000. The second, for use in the areas of dissection was less concentrated and was prepared with 20 mL of lidocaine 2%, 10 mL of ropivacaine 1%, and 470 mL of saline solution 0.9%, and epinephrine 1:500,000. Depending on the area of the face, part of these solutions was not used. The two sides of the face were infiltrated before the surgery was begun.
Intermittent pneumatic compression was used for prophylaxis of deep venous thrombosis during and after surgery until the patient began ambulation, usually 12 hours after surgery. Elastic stockings were used during surgery and until the fifteenth postoperative day. Subcutaneous enoxaparin was used in the preoperative, operative, and postoperative periods according to strict indications that were based upon personal risk factors and family history of deep venous thrombosis19,20.
The surgical procedure was performed as previously described21,22. Middle third rhytidoplasty was carried out, with a precapillary incision extended to the retroauricular area. The superficial musculoaponeurotic system (SMAS) in the middle third was treated with plication or resection, and sutures. No patient was treated with the confection of the SMAS flap. A lateral plication of the SMAS-platysma flap was performed. When indicated, endoscopic treatment of the frontal sinus and submental platysmaplasty were also performed. The order of execution in these cases was, first of all, frontal endoscopy, followed by treatment of the neck and, finally, by operation on the middle third.
The dissection in the neck and middle third, which was always wide, was performed with Metzenbaum scissors. In the neck, we tried to release the last inferior skin fold. The middle right third was executed before the left one. The limit of previous dissection in this region followed a longitudinal line, passing through the outer corner of the eye and making sure that there was an adequate release of dermal ligaments.
Hemostasis with electrocautery (Electrosurgical Generator SS-200A, WEM, Ribeirão Preto, SP, Brazil) was predominantly performed on larger vessels, preventing cauterization of the flap.
Proposed surgical procedures
After traction and fixation of the skin-fat flap on the right side and taking advantage of the left lateral position of the head, the construction of the hemostatic net was begun. This was performed with continuous sutures and focused on the compulsory closure of the virtual space generated during the dissection of the skin in the middle third and the neck. Mononylon 4-0 suture material (Ethilon 4-0, Ethicon, São José dos Campos, SP, Brazil) was used, with a triangular 30 mm needle. In cases of patients with very thin skin, alternatively, a 5-0 nylon suture can be use (5-0 monofilament nylon ethilon, Ethicon, São José dos Campos, SP, Brazil).
The first line of the net began in the area corresponding to the most medial and lower neck dissection, regardless of the submental route that had been used (Figure 1). The needle passage followed a uniform pattern, transfixing perpendicular to the skin, plunging into the SMAS-platysma at 45 degrees, and emerging at the same angle at a distance of 0.8 cm to 1 cm from the point of entry (Figure 2). The suture was continuous and a distance of 0.8 cm to 1 cm was maintained between stitches (Figure 1). This spacing and a mild traction on the thread by the assistant ensured that the suture would not be loose when the patient's head was turned medially. The SMAS-platysma was encompassed in each passage of the needle, so as to bring it into contact with the skin and thereby close the space.
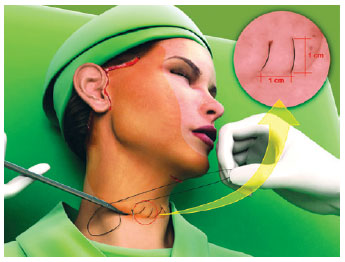
Figure 1 - The hemostatic net started at the lowest point at the base of the neck, ensuring a distance of 1 cm between the passages of the thread. The darker area corresponds to the detachment of the face.
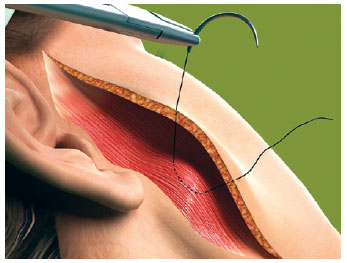
Figure 2 - The needle enters at 45 degrees, encloses the SMAS-platysma and emerges at 45 degrees.
The first line of suture ended in the more posterior portion of the retroauricular incision. The second line began about 1 cm above, following parallel to the first and so on, until all the unattached areas were covered by the hemostatic net (Figures 3 to 5).
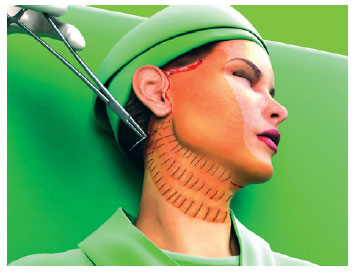
Figure 3 - The hemostatic net is established in parallel following a vertical orientation.
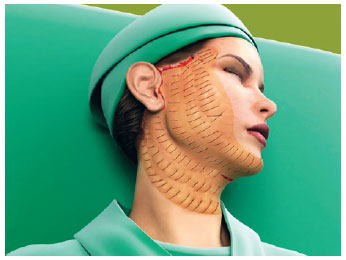
Figure 4 - The whole hemiface with the hemostatic net in place.
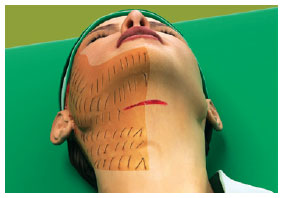
Figure 5 - The hemostatic net is also placed on the neck, ensuring closure of the detached area.
After the right side was finished, the left was dissected and pulled. The hemostatic net was made the same way as the opposite side.
Patients in group A received a Jackson-Pratt 15 F tubulolaminar drain (Jac-Cell Medic, Lachine, Canada) in each side of the face. Patients in group B, in whom the hemostatic net was used, were not drained.
The skin suture was made with surgical staples (Proximate, Ethicon Endo-Surgery, Cincinnati, United States) in the region above the projection of the mastoid process and retroauricular scalp, with 5-0 nylon stitches (Ethilon 5-0, Ethicon, São José dos Campos, SP, Brazil) in the retroauricular region and lobe, and with 4-0 poliglecaprone 25 sutures (Monocryl 4-0, Ethicon, São José dos Campos, SP, Brazil) in the preauricular and precapillary regions.
Patients were normally hospitalized for 48 hours. During this period, an occlusive dressing was maintained, and it was changed after 24 hours. On the second postoperative day, 2 to 3 hours before patient discharge, the bandage and the hemostatic net were removed. As with simple skin sutures, each loop of the net was cut and removed individually.
With the surgical procedure as described, it is natural to have some concerns about the quality of the scarring at the needle entry sites that create the hemostatic net. To assess the quality of scarring, patients in group B were evaluated at the end of the first and third postoperative months for the presence or absence of skin hyperpigmentation and hypopigmentation.
Statistical analysis
The results were analyzed using the Fisher exact test. P < 0.05 or 5% was established for rejection of the null hypothesis.
RESULTS
The average age of patients in group A was 55.4 years and ranged from 33 to 77 years, while that of group B was 54.1 years and ranged from 34 to 75 years. Most of the patients operated on were female (109 [90.8%] in group A and 229 [93.1%] in group B). Most of the patients were neither smokers nor hypertensive and were undergoing the surgery for the first time (Table 1).
Figures 6 to 11 illustrate the postoperative evolution of a 49-year-old patient who was submitted to rhytidoplasty, frontal videoendoscopy, upper and lower blepharoplasty, submental platysmaplasty, and rhinoplasty. The hemostatic net was used in this patient.
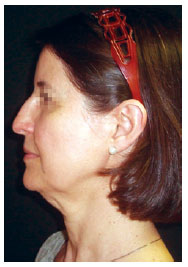
Figure 6 - Preoperative image. Surgical planning included rhytidectomy, frontal videoendoscopy, upper and lower blepharoplasty, submental platysmaplasty, and rhinoplasty. This patient underwent the hemostatic net procedure.
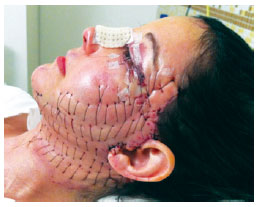
Figure 7 - Appearance of the patient 24 hours after surgery.
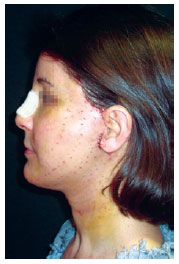
Figure 8 - Patient on the fourth postoperative day. Note the scabbing at the needle entry points.
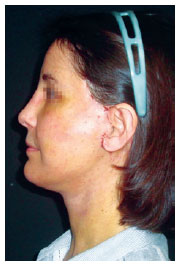
Figure 9 - Patient on the eighth postoperative day. Most of the needle entry points are already completely epithelialized.
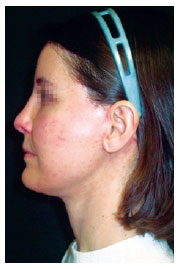
Figure 10 - Patient on the 24th postoperative day, with mild hyperpigmentation at the needle entry points.
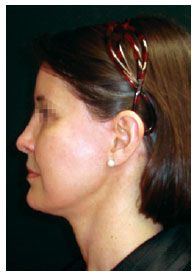
Figure 11 - Appearance of the patient in the fifth postoperative month.
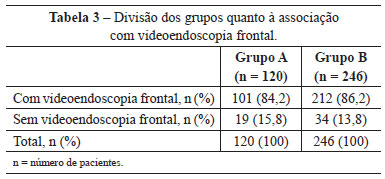
In group A, 102 (85%) patients were also submitted to submental platysmaplasty and 101 (84.2%), to frontal endoscopy. In group B, this correlation was observed in 233 (94.7%) and 212 (86.2%) patients, respectively (Tables 2 and 3).
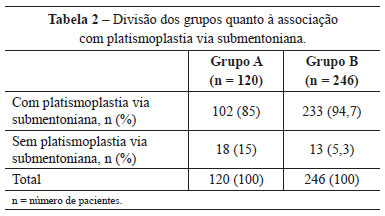
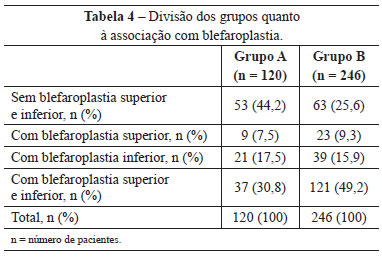
Upper and lower blepharoplasty was performed in 37 (30.8%) patients in group A and 121 (49.2%) in group B. Blepharoplasty was not performed in 53 (44.2%) patients in group A, and in 63 (25.6%) in group B (Table 4).
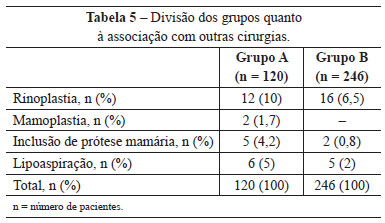
Other operations carried out simultaneously were also evaluated, and rhinoplasty was the most frequent (Table 5).
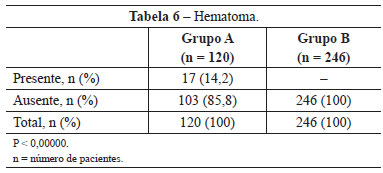
In group A, 17 (14.2%) patients developed hematomas in the first 72 hours. In group B, in which the hemostatic net was used, no patient developed this complication (P <0,00000) (Table 6).
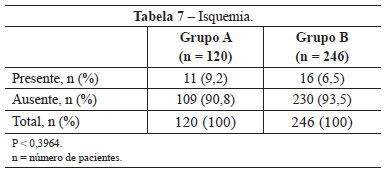
Ischemia was observed in 11 (9.2%) patients in group A, an incidence higher than that obtained in group B (6.5%; 16 patients). This difference was not statistically significant (P < 0.3964) (Table 7).
Necrosis was observed in 3 (2.5%) patients in group A and 4 (1.6%) in group B. This difference was not statistically significant (P < 0.4298) (Table 8). The images of two patients with similar cases of necrosis following rhytidoplasty, with and without the use of the hemostatic net, are shown in Figures 12 and 13, respectively.
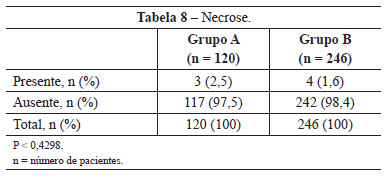
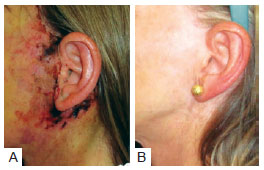
Figure 12 - In A, patient with necrosis in the periauricular region after rhytidoplasty without the use of the hemostatic net. In B, the same patient 2 years after surgery.
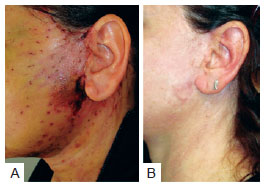
Figure 13 - In A, patient with necrosis in the periauricular region after rhytidoplasty with the hemostatic net. In B, the same patient 13 months after the surgery
Hyperpigmentation in the area of the sutures was observed in 42 (17.1%) patients who received the hemostatic net. This symptom resolved in an average of 3 months. In some patients, we used a bleaching solution of hydroquinone 2% after the first postoperative month, in order to accelerate recovery. Figure 14 presents the image of a patient who received this treatment: a month after surgery, hyperpigmentation developed in the region of the neck where the sutures had been applied. This hyperchromia disappeared after 3 months.
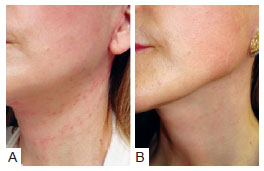
Figure 14 - Patient who underwent rhytidoplasty with the hemostatic net. In A, the patient 1 month postoperatively, showing hyperpigmentation on the neck. In B, the same patient 3 months postoperatively, with hyperpigmentation resolved.
Persistent hypopigmentation was found in 3 (1.2%) patients. The patient in Figure 15 illustrates this occurrence.
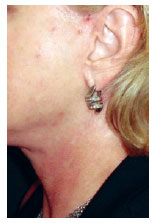
Figure 15 - Patient in the fourth postoperative month, showing areas of hypopigmentation in the region of the neck within the projection of the hemostatic net.
DISCUSSION
The occurrence of hematoma complicates the postoperative evolution of rhytidoplasty by leading to more edema and fibrosis1-4. In large hematomas, often found when the diagnosis is delayed more than is desirable, ischemia and necrosis of the skin-fat flap may occur and seriously compromise the outcome of surgery.
The occurrence of hematoma in the control group (14.2%), although high when compared to the incidences described by other authors (up to 8%), should be considered according to the magnitude of the detachment performed. While most published work indicates treatment of the lower levels of the neck, in this work, 85% of patients in the control group and 94.7% in the group with the hemostatic net were submitted to submental platysmaplasty. As was observed in a study that evaluated 1078 patients, this (submental platysmaplasty) is the single factor most associated with hematoma, with a relative risk of 4.3, ahead of arterial hypertension, gender, use of aspirin, and smoking23. In this study23, 26 (13.4%) of 194 patients who had previously undergone platysmaplasty developed hematomas, with an incidence that was similar to that of the present study.
Hematoma is a complication that typically generates stress not only for the patient but for the surgical team. It would not be an exaggeration to say that for many plastic surgeons, the realization of facial surgery without hematoma has become like a quest for the Holy Grail.
A solution has been found for seromas in abdominoplasties14,24. The use of internal sutures in abdominoplasties changed the way in which surgeons treated the operated region. The paradigm of using drains was transformed, especially for those who preferred simply not to use any drains.
Encouraged by the outcomes obtained with abdominoplasty sutures14 and inspired by the use of transfixing stitches for flap stabilization in rhytidoplasty15, the authors of this study began nearly 6 years ago to develop the idea of using a hemostatic net to prevent the development of hematomas. Previously, small hematomas were traditionally drained at the bedside, according to established procedures25,26. Large hematomas were eliminated in the operating room where drainage was achieved by opening the skin sutures and cauterizing the bleeding vessels.
This treatment was modified 6 years ago. Initially, recurrent and minor hematomas (localized areas of up to 5 cm in diameter) were treated by placement of transfixing sutures, to stabilize the skin and close the virtual space to avoid the formation of new deposits. The hematomas resolved without the occurrence of ischemia or necrosis and with good scarring evolution when the suture was applied and held for up to 48 hours.
Gaining confidence, the authors expanded the therapeutic use of these sutures, already in the form of a hemostatic net, to patients with large hematomas-those involving, for example, an entire side of the face. In keeping with this notion, 15 of the 17 patients in the control group of this study (group A) who developed hematomas were treated according to this procedure. This experience led the authors to a more daring step: to use the hemostatic net preventively and not only as a way to treat hematomas.
Thus, we established a routine for the surgical procedure, as described in this paper. The results indicate that the hemostatic net prevents the occurrence of hematoma in the first 72 postoperative hours. The mechanism that ensured this conclusion may be a combination of several factors such as the total obliteration of spaces, the pressure of the skin on the SMAS-platysma, and the stability of the flap.
Further, it was noted that the net did not cause a higher incidence of ischemia or necrosis, two complications that we naturally feared with the application of the suture to the flap. In this regard, several technical details should be considered. First of all, in the group with the net, exhaustive cauterization of the detached area was not necessary. This cauterization, especially for patients who bleed more, can compromise blood flow. Secondly, operations with extensive dissections, as were performed in this study, should ensure regular flaps, thick enough to maintain good perfusion. The vascular net is most often compromised in the regions of bone and musculocutaneous ligaments, as well as in the retroauricular region. Therefore, the professional experience of the surgeon might make a difference in the surgeon's ability to preserve the vascularization of the flap. For these reasons, it seems relevant that the procedure here proposed be used by surgeons that trust the quality of the flaps they need to dissect.
Although postoperative edema was not systematically evaluated, we found empirically an improvement in patients of group B when compared to those of group A. A future study may confirm this initial impression.
The question asked most often by patients and caregiver(s) relates to the unusual visual aspect of the homeostatic net and the possibility that it will leave permanent scars. The constant use of an occlusive dressing during the hospitalization period (48 hours) helps improve patient acceptance of the procedure and also contains small amounts of incisional bleeding.
With regard to the evolution of scarring from the sutures, it can be observed that, ever since the first cases in which the net was used as a treatment for hematoma, hyperpigmentation was not eliminated although transitional. The few patients with hypopigmentation did not suffer major repercussions, as it occurred mainly in the retroauricular region, in a barely noticeable manner.
CONCLUSIONS
The hemostatic net is an efficient method for the prevention of early hematomas in rhytidoplasty. This surgical procedure did not lead to a significant increase in the incidence of ischemia or necrosis.
REFERENCES
1. Baker TJ, Gordon HL. Complications of rhytidectomy. Plast Reconstr Surg. 1967;40(1):31-9.
2. Rees TD, Lee YC, Coburn RJ. Expanding hematoma after rhytidectomy: a retrospective study. Plast Reconstr Surg. 1973;51(2):149-53.
3. Stuzin JM. Face lifting. Plast Reconstr Surg. 2008;121(1 Suppl):1-19.
4. Baker DC, Stefani WA, Chiu ES. Reducing the incidence of hematoma requiring surgical evacuation following male rhytidectomy: a 30-year review of 985 cases. Plast Reconstr Surg. 2005;116(7):1973-85.
5. Huang TT, Blackwell SJ, Lewis SR. Routine use of a suction drain in facial rhytidoplasty. Ann Plast Surg. 1987;18(1):30-3.
6. Jones BM, Grover R, Hamilton S. The efficacy of surgical drainage in cervicofacial rhytidectomy: a prospective, randomized, controlled trial. Plast Reconstr Surg. 2007;120(1):263-70.
7. Marchac D, Sándor G. Face lifts and sprayed fibrin glue: an outcome analysis of 200 patients. Br J Plast Surg. 1994;47(5):306-9.
8. Fezza JP, Cartwright M, Mack W, Flaharty P. The use of aerosolized fibrin glue in face-lift surgery. Plast Reconstr Surg. 2002;110(2):658-64.
9. Kamer FM, Nguyen DB. Experience with fibrin glue in rhytidectomy. Plast Reconstr Surg. 2007;120(4):1045-51.
10. Por YC, Shi L, Samuel M, Song C, Yeow VK. Use of tissue sealants in face-lifts: a metaanalysis. Aesthetic Plast Surg. 2009;33(3):336-9.
11. Jones BM, Grover R. Reducing complications in cervicofacial rhytidectomy by tumescent infiltration: a comparative trial evaluating 678 consecutive face lifts. Plast Reconstr Surg. 2004;113(1):398-403.
12. Firmin FO, Marchac AC, Lotz NC. Use of the harmonic blade in face lifting: a report based on 420 operations. Plast Reconstr Surg. 2008;124(1):245-55.
13. Beer GM, Goldscheider E, Weber A, Lehmann K. Prevention of acute hematoma after face-lifts. Aesthetic Plast Surg. 2010;34(4):502-7.
14. Baroudi R, Ferreira CA. Seroma: how to avoid it and how to treat it. Aesthet Surg J. 1998;18(6):439-41.
15. Pontes R. O universo da ritidoplastia. Rio de Janeiro: Revinter; 2011.
16. Jones BM, Grover R. Avoiding hematoma in cervicofacial rhytidectomy: a personal 8-year quest. Reviewing 910 patients. Plast Reconstr Surg. 2004;113(1):381-7.
17. Gruber RP, Morley B. Ketamine-assisted intravenous sedation with midazolam: benefits and potential problems. Plast Reconstr Surg. 1999;104(6):1823-5.
18. Beninger FG, Pritchard SJ. Clonidine in the management of blood pressure during rhytidectomy. Aesthet Surg J. 1998;18(2):89-94.
19. Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg. 2004;114(3):43E-51E.
20. Durnig P, Jungwirth W. Low-molecular-weight heparin and postoperative bleeding in rhytidectomy. Plast Reconstr Surg. 2006;118(2):502-7.
21. Baker DC. Lateral SMASectomy. Plast Reconstr Surg. 1997;100(2):509-13.
22. McKinney P, Celetti S, Sweis I. An accurate technique for fixation in endoscopic brow litt. Plast Reconstr Surg. 1996;97(4):824-9.
23. Grover R, Jones BM, Waterhouse N. The prevention of haematoma following rhytidectomy: a review of 1078 consecutive facelifts. Br J Plast Surg. 2001;54(6):481-6.
24. Nahas FX, Ferreira LM, Ghelfond C. Does quilting suture prevent seroma in abdominoplasty? Plast Reconstr Surg. 2007;119(3):1060-4.
25. Pitanguy I, Ceravolo MP. Hematoma postrhytidectomy: how we treat it. Plast Reconstr Surg. 1981;67(4):526-9.
26. Baker DC, Chiu ES. Bedside treatment of early acute rhytidectomy hematomas. Plast Reconstr Surg. 2005;115(7):2119-22.
1. Plastic surgeon, master in surgery, full member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery) - SBCP, Curitiba, PR, Brazil.
2. Plastic surgeon, full member of SBCP, Curitiba, PR, Brazil.
3. Doctor in experimental surgery at the Universidade Federal de São Paulo (Federal University of São Paulo) - Unifesp, São Paulo, SP, Brazil; Associate professor at the Department of Surgery of the Universidade Federal do Paraná (Federal University of Paraná), Curitiba, PR, Brazil.
André Auersvald
Alameda Presidente Taunay, 1.756
Curitiba, PR, Brazil - CEP 82030-590
E-mail: andreauersvald@uol.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: August 10, 2011
Article accepted: November 28, 2011
Study conducted at Hospital Lipoplastic, Hospital de Cirurgia Estética e Reparadora, Curitiba, PR, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter