

Original Article - Year 2012 - Volume 27 -
Pharmacologic and intermittent pneumatic compression thromboembolic prophylaxis in 563 consecutives abdominoplasty cases
Profilaxia tromboembólica farmacológica e por compressão pneumática intermitente em 563 casos consecutivos de abdominoplastia
ABSTRACT
BACKGROUND: Abdominoplasty is a common cosmetic surgery and is subject to the same complications as any surgical procedure, including thromboembolic phenomena. The aim of this study was to assess the incidence of complications in consecutive abdominoplasties performed over a 3-year period, to identify risk factors for the complications, and to compare the efficacy of two protocols for prevention of thromboembolism.
METHODS: A retrospective study was conducted of 563 patients who underwent isolated abdominoplasty or abdominoplasty combined with additional cosmetic surgeries between March 2008 and April 2011. All patients received thromboembolism prophylaxis using either pharmacological (enoxaparin; 357 patients) or mechanical (intermittent pneumatic compression, IPC; 206 patients) protocols.
RESULTS: Of the 563 patients studied, 4 (0.7%) were male (0.7%) and 559 (99.3%) were female. The patients underwent isolated abdominoplasty (201; 35.7%) or abdominoplasty combined with other procedures (362; 64.3%). The patient groups receiving pharmacological and mechanical prophylaxis presented similar demographic and clinical characteristics and had similar risk factors for thromboembolic events. The incidence of complications in the patient groups undergoing pharmacological versus mechanical prophylaxis were: hematoma (5.6% vs. 10.7%), infection (2.2% vs. 2.4%), dehiscence (3.1% vs. 1.9%), seroma (2.2% vs. 2.4%), and deep vein thrombosis/pulmonary embolism (0.6% vs. 0.5%). There were no statistically significant differences in the incidence of complications between the two groups.
CONCLUSION: The incidence of complications in 563 consecutive cases of abdominoplasty was similar to that reported in the literature. The pharmacological and mechanical protocols for thromboembolic prophylaxis in abdominoplasty were equally effective.
Keywords: Abdomen/surgery. Venous thrombosis/prevention & control. Plastic surgery.
RESUMO
INTRODUÇÃO: A abdominoplastia é uma das cirurgias estéticas mais realizadas e, como qualquer outro ato cirúrgico, está sujeita a inúmeras complicações, entre as quais os fenômenos tromboembólicos. O objetivo deste estudo foi analisar a incidência de complicações em uma série consecutiva de abdominoplastias, fatores de risco e a eficácia de dois protocolos de prevenção para tromboembolia.
MÉTODO: Estudo retrospectivo de 563 abdominoplastias, isoladas ou não, realizadas entre março de 2008 e abril de 2011, que receberam dois protocolos de profilaxia de tromboembolismo diferentes: o farmacológico, com emprego de enoxaparina (357 pacientes), e o mecânico, com compressão pneumática intermitente (206 pacientes).
RESULTADOS: Dentre os 563 pacientes, 4 (0,7%) eram do sexo masculino (0,7%) e 559 (99,3%), do sexo feminino. Foram submetidos a abdominoplastia isolada 201 (35,7%) pacientes, enquanto 362 (64,3%) foram submetidos a abdominoplastia associada a algum outro procedimento. Os grupos com profilaxia farmacológica e mecânica tinham fatores de risco e características demográficas e clínicas semelhantes. A incidência de complicações no grupo farmacológico em relação ao grupo mecânico foi de: hematoma, 5,6% e 10,7%; infecção, 2,2% e 2,4%; deiscência, 3,1% e 1,9%; seroma, 2,2% e 2,4%; e trombose venosa profunda/tromboembolia pulmonar, 0,6% e 0,5%. Nenhuma complicação apresentou diferença estatística significante entre os grupos.
CONCLUSÕES: A taxa de complicações em 563 casos consecutivos de abdominoplastia foi semelhante à da literatura. A eficácia da profilaxia tromboembólica em abdominoplastia é a mesma observada com a utilização de métodos farmacológicos e mecânicos isoladamente.
Palavras-chave: Abdome/cirurgia. Trombose venosa/prevenção & controle. Cirurgia plástica.
All surgical procedures, including plastic surgery, carry the potential for complications such as infections, dehiscence, seroma, hematoma, scarring, thrombosis, embolism, and death. The incidence of complications from cosmetic surgery has been reported to be as high as 37%, although the vast majority of cases are not considered serious1. Given this, it is essential that patients are informed that complications can occur in until 25% of the cases2, and that it may be necessary to perform additional minor surgery to achieve the best aesthetic result (for example, to diminish unsightly scars and skin excess).
With the exception of death, the most troubling complication for plastic surgeons is thromboembolic events, which include deep venous thrombosis (DVT), pulmonary thromboembolism (PTE), and post-thrombotic syndrome3. In general surgery, the incidence of DVT varies from 16 to 30%4, the incidence of clinical manifestations of PTE is 1.6%, and death occurs in 0.1-0.8% of all surgeries5. PTE is considered the most preventable cause of death in hospitalized patients6.
Abdominoplasty is the most common cosmetic surgery and was first described at the end of the 19th century7. Since then, abdominoplasty has undergone several modifications such as liposuction, mini-abdominoplasty, conventional abdominoplasty, and lipo-abdominoplasty; these changes have improved the aesthetic result and decreased the incidence of complications8. The risk of complications from plastic surgery is increased by several risk factors such as obesity (BMI >30), systemic arterial hypertension (SAH), diabetes, smoking, patients undergoing bariatric surgery, surgical time, and combined surgeries1,9,10. The first complications related to abdominoplasty were described by Grazer and Goldwyn11, who reported incidences of 7.3% for infection, 5.4% for dehiscence, and 1.1% for DVT in a survey of surgeries performed by 958 surgeons on 10,490 patients.
As described in 1859 by the German physician Rudolf Virchow, thromboembolic events are caused by the combination of three factors: blood stasis, hypercoagulability, and endothelial damage12. Usually, DVT develops in the deep veins of the calf and can occur proximally to deep veins in up to 20% of cases, representing a serious risk in such patients13. About 50% of proximal DVT are associated with PTE, and 10% of these are fatal14. Therefore, prophylactic measures, early diagnosis, and appropriate treatments are essential to avoid tragedy in patients undergoing elective cosmetic surgery, who are usually healthy.
The typical signs of DVT include increased temperature, edema, severe calf pain, dilation of superficial veins, color change, and exacerbation of symptoms on lowering of the leg. The definitive diagnosis of DVT is made with Doppler ultrasound or lower limb venography. The diagnosis of PTE is suggested by symptoms such as dyspnea, ventilatory-dependent chest pain, and orthopnea (normally associated with DVT), and is confirmed by additional tests such as pulmonary scintigraphy and chest angiotomography.
Several options exist for thromboembolic prophylaxis, including early ambulation, pharmacological prophylaxis, or the use of physical interventions such as compression stockings, intermittent pneumatic compression (IPC), or vena cava filters15,16. Prophylactic measures have been highly successful in reducing the incidence of thromboembolic complications, although their individual mechanisms of action remain controversial. It is logical to think that IPC acts exclusively by physically increasing venous return and decreasing blood stasis17. However, studies have shown that IPC also lowers hypercoagulability by decreasing concentrations of inhibitors of plasminogen activation18,19, and this effect is enhanced when IPC is combined with pharmacological therapy20.
According to the literature, the incidence of DVT was reduced by 60%21 in patients undergoing IPC, 69% in patients receiving unfractionated heparin, and 78% in patients receiving low molecular weight heparin22. A recent meta-analysis suggests that mechanical and pharmacological therapies are more effective than isolated measures in preventing DVT and PTE in high-risk patients23. However, another meta-analysis failed to show that isolated mechanical prophylaxis reduced the incidence of DVT or PTE in patients with acute hemorrhagic cerebrovascular accident24. Nevertheless, mechanical prophylaxis should always be encouraged because no risk of bleeding or other contraindications have been identified25.
Drug prophylaxis has been reported to decrease the incidence of thromboembolic events following surgery by up to 70%26, although some studies suggest that it also increases the incidence of hematomas by up to 8%27, especially when large volumes and masses are removed28. The most commonly prescribed drugs for thromboembolic prophylaxis are warfarin, heparin and its derivatives, and antiplatelet medication. Warfarin is the most effective anticoagulant but there are several disadvantages to its use, such as the need to monitor blood levels, difficulty in reversing its effects, and the high rate of hematomas29. Antiplatelet medications, particularly acetylsalicylic acid, also play a role in the prevention of thromboembolic events, although to a lesser extent than heparin29. When used in conjunction with IPC devices, however, acetylsalicylic acid has the same anti-thromboembolic efficacy as heparin30. Although they are associated with bleeding complications, unfractionated and fractionated heparins are the most studied anticoagulants and the most commonly used in daily practice31.
The first large study of complication rates for abdominoplasties performed in the United States was published in 200932. The analysis used the national databases TOPS (database of the American Society of Plastic Surgery) and CosmetAssure (database of the insurers) to evaluate 20,970 abdominoplasties performed in combination with other surgeries and 10,660 isolated abdominoplasties32. The most prevalent complications in this study were hematomas (0.5%-0.9%), DVT/PTE (0.1-0.4%), and infections (0.3-3.5%), although the incidences varied between the databases and among the combined and isolated surgeries. The importance of these data lies in their reflection of "real life" practices, because there was no bias for academic environments, individual surgeons, or single institutions32. These types of bias have been observed in studies of other procedures; for instance, carotid endarterectomy surgery33.
Few studies in the literature address the incidence of DVT and PTE in patients undergoing plastic surgery, regardless of the type of procedure performed34. Surprisingly, the fact that these complications carry a high risk of morbidity and mortality does not make surgeons less resistant to the use of prophylactic measures. This resistance appears to derive from the assumption that prophylactic measures may cause higher incidences of hemorrhagic complications or that complications such as DVT and PTE are too rare to be of concern35. Studies in the United States show that 25.2% of surgeons do not recommend any form of prophylaxis in patients undergoing liposuction or abdominoplasty, and 18.4% do not recommend prophylaxis in patients undergoing facelifts34,36. In contrast, we have developed specific prophylaxis protocols that are implemented in many hospitals and clinics in Brazil, including at the Ivo Pitanguy Clinic since 2007 37.
As part of an attempt to stratify the risk of a patient developing DVT/PTE, we created protocols to guide the decision to administer prophylaxis and to select the best therapeutic option for each individual case. A previous study attempted to define the need for drug prophylaxis by identifying risk factors for thromboembolic events38. For plastic surgery in Brazil, one of the most common protocols is based on the work of Anger et al.38, in which points are assigned to known risk factors for thromboembolic events, and drug prophylaxis is recommended based on the total scores. The risk factors are shown in Clinical Features 1. Patients with scores of <1 are considered at low risk for thromboembolic events and do not require prophylaxis. Patients with scores between 2 and 4 are considered at moderate risk, and those with scores of >5 are at high risk. Pharmacological prophylaxis is indicated for all patients with scores >2.
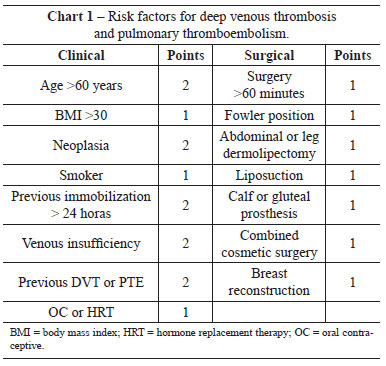
For abdominoplasty, the incidence of PTE and DVT has been reported to range from 0.34% to 1.1%11,39-41 for PTE and 0.1% to 0.34%3,32,40 for DVT. Several risk factors predispose to DVT, the most relevant being obesity (BMI >30)42. In practice, all patients undergoing abdominoplasty are considered at least at moderate risk (1 point for surgery of more than 60 minutes duration and 1 point for the surgery itself). Therefore, all patients submitted to abdominoplasty should receive thromboembolic prophylaxis.
Several protocols exist for PTE/DVT prevention, such as the protocols of Davison-Caprini43 (commonly used in the United States), Sandri44, and the modified version of Sandri45 (more common in Brazil and very similar to the protocol of Anger). The Davison-Caprini protocol recommends high-risk patients receive 40 mg enoxaparin delivered subcutaneously at 12 hours after surgery. Several studies have attempted to improve the Davison-Caprini protocol by taking into consideration additional plastic surgery-related risk factors, such as the use of hormone replacement therapy (HRT) and oral contraceptives (OC), BMI >30, and circumferential abdominoplasty46. The protocol of Sandri44 recommends that moderate- and high-risk patients receive the same pharmacological prophylaxis as in the Davison-Caprini protocol. A significant difference between the two protocols is that the protocol of Sandri44 includes risk factors common to cosmetic surgeries, such as the Fowler position, abdominal or crural dermolipectomy, gluteal and calf implants, combined cosmetic surgery, and liposuction. The modified protocol of Sandri45 includes 3 risks factors (general anesthesia, malignancy, bed rest of >72 hours) and recommends pharmacological prophylaxis for high-risk patients only, starting 12 hours after the procedure45. It is interesting to note that these three protocols have a degree of recommendation of two, and a level of evidence of C47, the highest score that can be given to studies that are not randomized and controlled.
Although abdominoplasty has a low mortality rate overall, the leading cause of death from this surgery is PTE. However, the relationship between PTE and abdominoplasty has still to be elucidated by surgeons48. Compared to other plastic surgeries, there are abdominoplasty-specific procedures that might be considered additional risk factors for PTE, such as plication of the abdominal muscles with increased intra-abdominal pressure, or development of abdominal compartment syndrome49. This effect might also be caused by the use of tight elastic compressive meshes50.
Because there is little literature on the relationship between abdominoplasty and thromboembolic events, we analyzed several characteristics of a patient population who underwent abdominoplasty from March 2008 to April 2011, including the complications from surgery, in particular thromboembolic events, and the possible risk factors. We also analyzed the incidence of complications in surgeries using two different protocols for thromboembolism prevention; one pharmacological and one mechanical.
METHODS
A retrospective study was carried out by analyzing the medical records of all patients undergoing consecutive abdominoplasty between March 2008 and April 2011. The cases included patients undergoing abdominoplasty either alone or in combination with other procedures. No patients were excluded from the analysis.
The patients were categorized as those who underwent pharmacological thromboembolic prophylaxis alone or those who underwent mechanical prophylaxis with IPC alone. No patients received combined therapy.
According to the classification published by Anger et al.38, all patients undergoing abdominoplasty have at least a moderate risk of thromboembolic events (score >2). Therefore, all patients received prophylaxis.
Patients operated on between March 2008 and April 2010 received pharmacologic therapy, and those operated on between May 2010 and April 2011 received mechanical therapy with IPC.
For drug prophylaxis, enoxaparin (Clexane®) was administered at a dose of 0.5 mg/kg body weight, starting 12 hours after surgery and continuing once a day until the patient was ambulatory. For mechanical prophylaxis with IPC, pneumatic compression of feet and calves was performed (DVT Phlebopress®) with pressure varying between 50 and 60 mmHg, starting before the induction of anesthesia and continuing until the patient was ambulatory.
The surgical technique employed was a typical abdominoplasty with horizontal incision above the pubic hair, detachment of the aponeurosis to the xiphoid appendix, plication of the rectus abdominis muscles, repositioning of the umbilical scar, drainage with closed system vacuum, lipectomy of the abdominal flap, and synthesis of the skin by planes. In specific cases, liposuction of the epigastric region and flanks was performed, as indicated by aesthetics.
Statistical analysis was carried out using IBM SPSS software version 19.0. The data were analyzed using two-tailed tests with the level of significance set at P < 0.05.
This work was approved by the Ethics Committees of the hospitals where the surgeries were performed.
RESULTS
Between March 2008 and April 2011, 563 patients underwent abdominoplasty, 4 (0.7%) of which were males and 559 (99.3%) females.
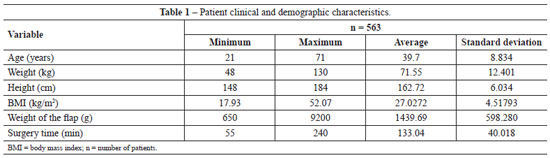
Table 1 provides the details of patient age, weight, height, and BMI, as well as the weight of the excised abdominal flap and surgery time.
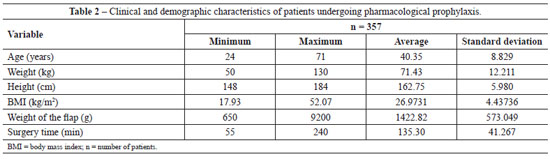
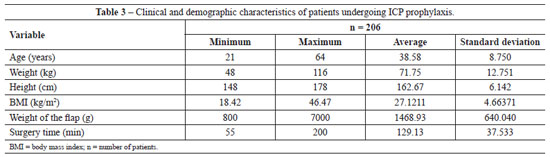
All patients (n=357) undergoing abdominoplasty between March 2008 and April 2010 received pharmacological thromboembolic prophylaxis, and patients operated on between May 2010 and April 2011 (n=206) received mechanical prophylaxis with IPC. The descriptive statistics of the two groups is presented in Tables 2 and 3.
Analysis of the data in Tables 2 and 3 shows non-parametric distribution of the patient populations. Using the Mann-Whitney U test to compare the groups receiving pharmacological and IPC prophylaxis, there were no significant differences in age (P = 0.089), weight (P = 0.971), height (P = 0.811), weight of tissue removed (P = 0.545), or surgery time (P = 0.087).
The patient risk factors that we considered might predispose to complications, especially thromboembolic events, included undergoing combined surgery, surgery time, obesity (BMI >30), and a history of smoking. Of the 563 patients, only 201 (35.7%) underwent isolated abdominoplasty, whereas 362 (64.3%) underwent abdominoplasty combined with other procedures (Table 4). The types of combination surgeries performed were similarly distributed in the patient groups receiving pharmacological and mechanical IPC prophylaxis (Figures 1 and 2).
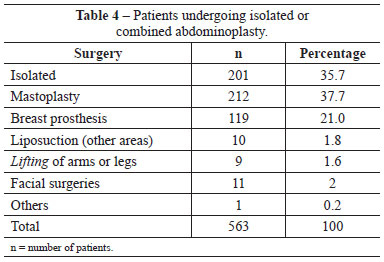
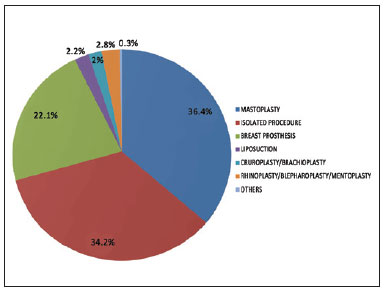
Figure 1 - Distribution of combined surgeries in the population undergoing pharmacological prophylaxis.
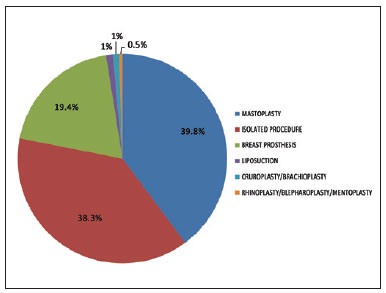
Figure 2 - Distribution of combined surgeries in the population undergoing ICP prophylaxis.
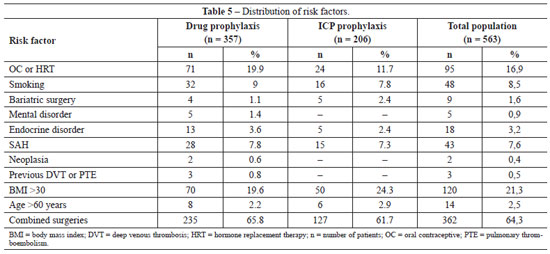
The distributions of risk factors for thromboembolic events in the general population and in the two treatment groups are shown in Table 5. There was only one significant difference in risk factors between the groups, with a higher frequency of OC use in the group subjected to ICP prophylaxis (chi-square test; P = 0.012). No other significant differences in risk factors were detected (chi-square test; smoking, P = 0.754; previous bariatric surgery, P = 0.299; mental disorders, P = 0.164; endocrine disorders, P = 0.430; SAH, P = 0.809; neoplasia, P = 0.282; and previous DVT/PTE, P = 0.187).
No patient had venous insufficiency, previous immobilization exceeding 24 hours, breast reconstruction, or gluteal or calf prostheses, which are common risk factors for developing thromboembolic events according to the studies of Anger et al.38.
Only three patients were in surgery for less than 60 minutes (two in the pharmacological prophylaxis group and one in the ICP group). However, all three patients were using OC and one was a smoker, which placed them at moderate risk and indicated the use of DVT/PTE prophylaxis.
Only the group subjected to pharmacological prophylaxis contained patients with two serious risk factors for the occurrence of thromboembolic events; namely, the presence of neoplasia (2 patients) and previous DVT or PTE (3 patients). However, none of these patients developed new thromboembolic events after surgery, demonstrating the efficacy of the prophylaxis.
We also studied the correlation between thromboembolic events and the presence of some risk factors not generally considered to increase the incidence of DVT/PTE, including SAH, previous bariatric surgery, mental disorders (depression, anxiety, epilepsy), and endocrine disorders (diabetes mellitus, hypothyroidism). Among these risk factors, only the presence of previous bariatric surgery has previously been associated with an increased risk for thromboembolic events.
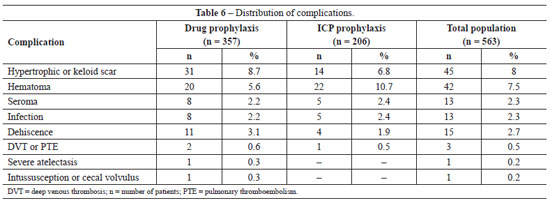
The incidence of post-operative complications in the general population and in groups undergoing pharmacological and ICP prophylaxis is shown in Table 6. Using chi-square analysis, no significant differences were observed between the two groups in the incidence of any complication: infection, P = 0.977; seroma, P = 0.977; DVT/PTE, P = 0.991; dehiscence, P = 0.716; and hypertrophic or keloid scar, P = 0.568. The only complication showing a difference, albeit not statistically significant (chi-square; P = 0.080), was the higher occurrence of hematoma in the ICP prophylaxis group. Despite the lower incidence of hematomas in the pharmacological prophylaxis group, the severity of the hematomas was higher. Thus, of the 20 hematomas observed in the pharmacological prophylaxis group, 6 required surgical re-intervention and 2 patients required blood transfusions. In contrast, only 2 of the 22 hematomas occurring in the ICP prophylaxis group required surgical re-intervention and neither patient required blood transfusion.
Two unusual complications were observed in the group subjected to pharmacological prophylaxis; severe pulmonary atelectasis, and intussusception and volvulus of the cecum. In the first case, the patient required admission to the intensive care unit for clinical pulmonary compensation. After examination and evaluation by the thoracic surgery and pneumology team, the possibility of PTE was excluded and the patient was treated for severe pulmonary atelectasis, with complete resolution of symptoms. In the case of intussusception and volvulus of the cecum, the patient showed signs of acute intra-abdominal pathology 30 days after abdominoplasty and imaging examinations suggested the diagnosis of intussusception and volvulus of the cecum. The patient underwent exploratory laparotomy using the previous abdominoplasty incision and the suspected condition was confirmed by visual examination. The case achieved a good outcome. This complication, although rare, has previously been associated with abdominoplasty51.
The three patients experiencing DVT or PTE all recovered. These patients had undergone abdominoplasty combined with other cosmetic surgeries and had thromboembolism risk scores of >5, making them high risk. In the pharmacological prophylaxis group, one patient had isolated DVT and the second had DVT followed by moderate PTE, which required intensive care measures. The single patient in the ICP prophylaxis group had DVT followed by mild PTE, which did not require intensive therapy. No patient developed post-thrombotic syndrome and there were no deaths.
DISCUSSION
Plastic surgery is an easy target for criticism in the media and is the subject of many legal battles. Many people consider that because it is elective, cosmetic surgery should be free of complications and should achieve pleasing results regardless of the procedure employed. Moreover, the expectations of the patient should always be met as stated in the Consumer Protection Code. These interpretations, in addition to being misleading, question the medical and scientific knowledge on which the specialty is based, and make plastic surgeons easy prey for the media and legal system. Every surgery has the potential for complications, as amply demonstrated in the world medical literature. The cases presented in this work are no different.
Apart from patient mortality, the greatest concern of surgeons is the occurrence of thromboembolic events, which is considered to be the Achilles heel during this type of surgery. The pathophysiology of these phenomena was described by Virchow, who assigned blood stasis, damage to the endothelium, and hypercoagulability as the primary factors in thrombosis. All three factors can be exacerbated by surgery, abdominoplasty in particular, due to the prolonged immobilization, surgical position, damage to the endothelium, and production of procoagulant factors (cytokines and hormones)13,52. Several additional risk factors such as smoking, obesity, venous insufficiency, neoplasia, age, and duration of surgery may further increase the possibility of developing thromboembolic events38,43.
This study presents a retrospective analysis of 563 patients who underwent abdominoplasty in combination with either pharmacological (enoxaparin) or mechanical (ICP) prophylaxis for the prevention of thromboembolic events. Several prophylaxis protocols already exist, usually pharmacological, and should be recommended to all patients undergoing abdominoplasty because the surgical time and post-operative position constitute a moderate risk of thromboembolic events38,43. However, plastic surgeons have shown strong resistance to perform pharmacological prophylaxis. Studies show that up to 25% of plastic surgeons do not recommend any type of prophylaxis for patients undergoing abdominoplasty34, either because they fear hemorrhagic complications or consider thromboembolic events too rare to be of concern34. Thus, it is important to develop protocols and safety guidelines for prophylaxis of thromboembolism in patients undergoing abdominoplasty53.
It is interesting to note that in the present study, 64.3% of abdominoplasties were performed in combination with other cosmetic procedures. This practice is increasingly common in plastic surgery because it is more convenient for both the doctor and patient. However, combination surgery increases the occurrence of thromboembolic events54; not only should this be explained to the patient, but it should also mandate the use of DVT and PTE prophylaxis.
The complications found in this study were hypertrophic scar or keloid (8.0%), hematoma (7.5%), seroma (2.3%), infection (2.3%), dehiscence (2.7%), and DVT/PTE (0.5%). We also observed a case of severe pulmonary atelectasis requiring intensive support, and a case of intussusception and volvulus of the cecum, which has been previously described and associated with abdominoplasty51.
The previously reported incidences of these complications following abdominoplasty are: keloid or hypertrophic scars 5-16%55-57, hematomas 0.5-8.0%2,32,37,58, seroma 1.0-8.0%3,26, infection 2.2-7.3%11,32, dehiscence 0.7-5.4%3,11, and DVT/PTE 0.5-1.1%11,32,39. However, the incidence of DVT/PTE can reach 6.6% when abdominoplasty is combined with other procedures59.
The data presented in this study are consistent with those in the literature, except that we observed a higher incidence of hematoma in the ICP prophylaxis population (10.7%). In contrast, the rate of hematoma in the pharmacological prophylaxis group (5.6%) was similar to that reported in the literature. The incidence of bleeding and hemorrhagic complications is known to be increased by pharmacological prophylaxis31, but not by mechanical prophylaxis17,19 as was observed in our study. We cannot explain the higher incidence of hematoma in the ICP prophylaxis group, although it should be noted that the incidence between the two groups was not statistically different (chi-square; P = 0.08). Moreover, the bleeding complications in the pharmacological prophylaxis group were more severe and required more frequent surgical re-intervention and hemotherapy, demonstrating an advantage to the use of ICP mechanical prophylaxis.
The incidence of thromboembolic events in our study was 0.6% in the pharmacological prophylaxis group, 0.5% in the ICP prophylaxis group, and 0.5% in the general population, which is similar to the literature worldwide3,11,32. The results show that thromboembolic complications do occur following abdominoplasty, albeit at low incidence. The high rates of morbidity and mortality associated with these events necessitates that prophylactic measures should be taken for every surgery.
This is the first report to compare pharmacological and mechanical protocols for prophylaxis of thromboembolic events in patients undergoing plastic surgery, and specifically abdominoplasty. We found that the prophylactic methods were equally effective in preventing DVT/PTE, and either one can be used in isolation for this purpose.
CONCLUSION
The incidence of complications observed in this study is similar to that reported in the literature. ICP mechanical prophylaxis is as effective as pharmacological prophylaxis in preventing thromboembolic events in patients undergoing abdominoplasty. Although the incidence of hemorrhagic phenomena was the same in both groups of patients, the events were less severe in patients receiving ICP mechanical prophylaxis.
REFERENCES
1. Neaman KC, Hansen JE. Analysis of complications from abdominoplasty: a review of 206 cases at a university hospital. Ann Plast Surg. 2007;58(3):292-8.
2. Stewart KJ, Stewart DA, Coghlan B, Harrison DH, Jones BM, Waterhouse N. Complications of 278 consecutive abdominoplasties. J Plast Reconstr Aesthet Surg. 2006;59(11):1152-5.
3. Jatene PRS, Jatene MCV, Barbosa ALM. Abdominoplastia: experiência clínica, complicações e revisão da literatura. Rev Soc Bras Cir Plást. 2005;20(2):65-71.
4. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients: Results of meta-analysis. Ann Surg. 1988; 208(2):227-40.
5. McDevitt NB. Deep vein thrombosis prophylaxis. American Society of Plastic and Reconstructive Surgeons. Plast Reconstr Surg. 1999; 104(6):1923-8.
6. Michota FA. Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22(12):1762-70.
7. Kelly HA. Excision of the flat abdominal wall lipectomy. Surg Gynec Obstet. 1910;10:229-31.
8. Pitanguy I. Evaluation of body contouring surgery today: a 30-year perspective. Plast Reconstr Surg. 2000;105(4):1499-516.
9. Vastine V, Morgan RF, Williams GS, Gampper TJ, Drake DB, Knox LK, et al. Wound complications of abdominoplasty in obese patients. Ann Plast Surg. 1999;42(1):34-9.
10. Hensel JM, Lehman JA Jr, Tantri MP, Parker MG, Wagner DS, Topham NS. An outcomes analysis and satisfaction survey of 199 consecutive abdominoplasties. Ann Plast Surg. 2001;46(4):357-63.
11. Grazer FM, Goldwyn RM. Abdominoplasty assessed by survey, with emphasis on complications. Plast Reconstr Surg. 1977;59(4):513-7.
12. Risberg B. Pathophysiological mechanisms of thromboembolism. Acta Chir Scand Suppl. 1988;550:104-14.
13. Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I22-30.
14. Hull RD, Pineo GF. Treatment of venous thromboembolism with low molecular weight heparins. Hematol Oncol Clin North Am. 1992;6(5):1095-103.
15. Caprini JA. Intermittent pneumatic compression and pharmacologic thrombosis prophylaxis. Curr Opin Pulm Med. 2009;15(5):439-42.
16. Kaboli P, Henderson MC, White RH. DVT prophylaxis and anticoagulation in the surgical patient. Med Clin North Am. 2003;87(1):77-110.
17. Cahan MA, Hanna DJ, Wiley LA, Cox DK, Killewich LA. External pneumatic compression and fibrinolysis in abdominal surgery. J Vasc Surg. 2000;32(3):537-43.
18. Comerota AJ, Chouhan V, Harada RN, Sun L, Hosking J, Veermansunemi R, et al. The fibrinolytic effects of intermittent pneumatic compression: mechanism of enhanced fibrinolysis. Ann Surg. 1997;226(3):306-14.
19. Chen AH, Frangos SG, Kilaru S, Sumpio BE. Intermittent pneumatic compression devices: physiological mechanisms of action. Eur J Vasc Endovasc Surg. 2001;21(5):383-92.
20. Kiudelis M, Gerbutavicius R, Gerbutaviciene R, Griniute R, Mickevicius A, Endzinas Z, et al. A combinative effect of low-molecular-weight heparin and intermittent pneumatic compression device for thrombosis prevention during laparoscopic fundoplication. Medicina (Kaunas, Lithuania). 2010;46(1):18-23.
21. Jeffery PC, Nicolaides AN. Graduated compression stockings in the prevention of postoperative deep vein thrombosis. Br J Surg. 1990;77(4):380-3.
22. Leyvraz PF, Bachmann F, Hoek J, Büller HR, Postel M, Samama M, et al. Prevention of deep vein thrombosis after hip replacement: randomised comparison between unfractionated heparin and low molecular weight heparin. BMJ. 1991;303(6802):543-8.
23. Kakkos SK, Caprini JA, Geroulakos G, Nicolaides AN, Stansby GP, Reddy DJ. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thrombo-embolism in high-risk patients. Eur J Vasc Endovasc Surg. 2009;37(3):364-5.
24. Naccarato M, Chiodo Grandi F, Dennis M, Sandercock PA. Physical methods for preventing deep vein thrombosis in stroke. Cochrane Database Syst Rev. 2010;(8):CD001922.
25. Lippi G, Favaloro EJ, Cervellin G. Prevention of venous thromboembolism: focus on mechanical prophylaxis. Semin Thromb Hemost. 2011;37(3):237-51.
26. Saldanha OR, De Souza Pinto EB, Mattos WN Jr, Pazetti CE, Lopes Bello EM, Rojas Y, et al. Lipoabdominoplasty with selective and safe undermining. Aesthetic Plast Surg. 2003;27(4):322-7.
27. Elias A, Fiessinger JN. Maladie thromboembolique veineuse. Paris: Masson; 1995.
28. Geller DS, Hornicek FJ, Mankin HJ, Raskin KA. Soft tissue sarcoma resection volume associated with wound-healing complications. Clin Orthop Relat Res. 2007;459:182-5.
29. Conduah A, Lieberman JR. Venous thromboembolic prophylaxis after elective total hip arthroplasty. Clin Orthop Relat Res. 2005;441:274-84.
30. Patel AR, Crist MK, Nemitz J, Mayerson JL. Aspirin and compression devices versus low-molecular-weight heparin and PCD for VTE prophylaxis in orthopedic oncology patients. J Surg Oncol. 2010;102(3):276-81.
31. Koch A, Bouges S, Ziegler S, Dinkel H, Daures JP, Victor N. Low molecular weight heparin and unfractionated heparin in thrombosis prophylaxis after major surgical intervention: update of previous meta-analyses. Br J Surg. 1997;84(6):750-9.
32. Alderman AK, Collins ED, Streu R, Grotting JC, Sulkin AL, Neligan P, et al. Benchmarking outcomes in plastic surgery: national complication rates for abdominoplasty and breast augmentation. Plast Reconstr Surg. 2009;124(6):2127-33.
33. Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA. 1998;279(16):1278-81.
34. Broughton G 2nd, Rios JL, Rohrich RJ, Brown SA. Deep venous thrombosis prophylaxis practice and treatment strategies among plastic surgeons: survey results. Plast Reconstr Surg. 2007;119(1):157-74.
35. Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, et al. Prevention of venous thromboembolism. Chest. 2001;119(1 Suppl):132S-75S.
36. Young VL, Watson ME. The need for venous thromboembolism (VTE) prophylaxis in plastic surgery. Aesthetic Surg J. 2006;26(2):157-75.
37. Paiva RA, Pitanguy I, Amorim NFG, Berger R, Shdick HA, Holanda TA. Tromboembolismo venoso em Cirurgia Plástica: protocolo de prevenção na Clínica Ivo Pitanguy. Rev Bras Cir Plást. 2010;25(4):583-8.
38. Anger J, Baruzzi ACA, Knobel E. Um protocolo de prevenção de trombose venosa profunda em Cirurgia Plástica. Rev Soc Bras Cir Plást. 2003;18(1):47-54.
39. Matarasso A, Swift RW, Rankin M. Abdominoplasty and abdominal contour surgery: a national plastic surgery survey. Plast Reconstr Surg. 2006;117(6):1797-808.
40. Almeida EG, Almeida Jr GL. Abdominoplastia: estudo retrospectivo. Rev Soc Bras Cir Plast. 2008;23(1):1-10.
41. Flinn WR, Sandager GP, Silva Jr MB, Benjamin ME, Cerullo LJ, Taylor M. Prospective surveillance for perioperative venous thrombosis: experience in 2643 patients. Arch Surg. 1989;131(5):472-80.
42. Murphy RX Jr, Peterson EA, Adkinson JM, Reed JF 3rd. Plastic surgeon compliance with national safety initiatives: clinical outcomes and "never events". Plast Reconstr Surg. 2010;126(2):653-6.
43. Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg. 2004;114(3):43E-51E.
44. Sandri JL. Profilaxia do tromboembolismo em cirurgia plástica. In: Carreirão S, Cardim V, Goldenberg D, eds. Cirurgia Plástica - Sociedade Brasileira de Cirurgia Plástica. São Paulo: Atheneu; 2005. p. 119-25.
45. Moulim JL, Sobreira ML, Malgor RD, Abreu CR, Araújo ESF, Palhares Neto AA. Estudo comparativo entre protocolos para profilaxia da trombose venosa profunda: uma nova proposta. Rev Bras Cir Plást. 2010;25(3):415-22.
46. Hatef DA, Kenkel JM, Nguyen MQ, Farkas JP, Abtahi F, Rohrich RJ, et al. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122(1):269-79.
47. Guyatt G, Schunemann HJ, Cook D, Jaeschke R, Pauker S. Applying the grades of recommendation for antithrombotic and thrombolytic therapy: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):179S-87S.
48. Keyes GR, Singer R, Iverson RE, McGuire M, Yates J, Gold A, et al. Mortality in outpatient surgery. Plast Reconstr Surg. 2008;122(1):245-53.
49. Graça Neto L, Araújo LR, Rudy MR, Auersvald LA, Graf R. Intraabdominal pressure in abdominoplasty patients. Aesthetic Plast Surg. 2006;30(6):655-8.
50. Clayman MA, Clayman ES, Seagle BM, Sadove R. The pathophysiology of venous thromboembolism: implications with compression garments. Ann Plast Surg. 2009;62(5):468-72.
51. Destro MWB, Destro C, Salles VJA, Cauduro AB, Kalume RS. Volvo de ceco no pós-operatório recente de abdominoplastia. Rev Soc Bras Cir Plast. 2007;22(3):176-9.
52. Kroegel C, Reissig A. Principle mechanisms underlying venous thromboembolism: epidemiology, risk factors, pathophysiology and pathogenesis. Respiration. 2003;70(1):7-30.
53. Miszkiewicz K, Perreault I, Landes G, Harris PG, Sampalis JS, Dionyssopoulos A, et al. Venous thromboembolism in plastic surgery: incidence, current practice and recommendations. J Plast Reconstr Aesthet Surg. 2009;62(5):580-8.
54. Sellam P, Trevidic P. The thromboembolic risk in abdominal plastic surgery. A randomized statistical study of 190 cases. Ann Chir Plast Esthet. 1999;44(5):545-8.
55. Chike-Obi CJ, Cole PD, Brissett AE. Keloids: pathogenesis, clinical features, and management. Semin Plast Surg. 2009;23(3):178-84.
56. Aköz T, Gideroğlu K, Akan M. Combination of different techniques for the treatment of earlobe keloids. Aesthetic Plast Surg. 2002;26(3):184-8.
57. Alves JCRR, Silva Filho AF, Pereira NA. Cicatrização patológica e seu tratamento. In: Melega JM, ed. Cirurgia Plástica: fundamentos e arte. São Paulo: MEDSI; 2002. p. 15-24.
58. Abs R. Accidents thromboemboliques en chirurgie plastique: revue de la literature et proposition d´un algorithme de prophylaxie. Ann Chir Plast Esthet. 2000;45(6):604-9.
59. Aly AS, Cram AE, Chao M, Pang J, McKeon M. Belt lipectomy for circumferential truncal excess: the University of Iowa experience. Plast Reconstr Surg. 2003;111(1):398-413.
1. Plastic Surgeon, member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery), Ribeirão Preto, SP, Brazil.
2. Anesthesiologist, member of the Sociedade Brasileira de Anestesiologia (Brazilian Society of Anesthesiology), Ribeirão Preto, SP, Brazil.
3. Doctor and assistant researcher in Neuroscience at the Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (Faculty of Medicine of Ribeirão Preto) - FMRP-USP, Ribeirão Preto, SP, Brazil.
Endrigo Piva Pontelli
Rua Carlos Rateb Cury, 697 - casa 61 - Bonfim Paulista - Cond. Vila Buenos Aires
Ribeirão Preto, SP, Brazil - CEP 14110-000
E-mail: epontelli@bol.com.br
Paper submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: May 23, 2011
Article accepted: November 11, 2011
Study conducted at the Hospital Santa Lydia e Hospital da Plástica de Ribeirão Preto (Santa Lydia Hospital and Hospital da Plástica of Ribeirão Preto), Ribeirão Preto, SP, Brazil.
Work submitted in consideration for promotion to full member of BSPS.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter