

Original Article - Year 2012 - Volume 27 -
Keloid and hypertrophic scar distribution according to Fitzpatrick skin phototypes
Distribuição de queloide e cicatriz hipertrófica segundo fototipos de pele de Fitzpatrick
ABSTRACT
BACKGROUND: Keloid and hypertrophic scars have a common physiopathogenic origin and are defined as fibroproliferative scars. Fibroproliferative scars are frequent in individuals with darker skin. However, mixing of "races" renders it difficult to group patients with different skin tones according to morphological and static classifications (white for Caucasians; brown for individuals of Spanish descent (Hispanic/Latino); yellow for individuals of East Asian descent; and black for individuals of African descent) according to their response to sun exposure. It is known that when individuals whose ethnic origin is in colder countries move to tropical countries, they show a higher incidence of these types of scars, which mainly affect parts of the body that are more exposed to the sun. A correlation between fibroproliferative scars and Fitzpatrick phototype, a dynamic classification based on the skin's response to sun exposure, would contribute to an understanding of the pathophysiology of these scars. The aim of this study is to investigate the distribution of fibroproliferative scars according to Fitzpatrick phototypes.
METHODS: We classified patients' fibroproliferative scars according to the Muir classification as Long-Term Evolution (keloid scars), Short-Term Evolution (hypertrophic scars), and Intermediate Group (mixed scars), while their skin types were grouped according to the Fitzpatrick classification.
RESULTS: Fitzpatrick phototype III and mixed scars were predominant among the patients analyzed (p = 0.001). A correlation (p = 0.025) was observed between fibroproliferative scars and Fitzpatrick phototypes; the higher the phototype, the higher the tendency to develop keloid and mixed scar tissue.
CONCLUSIONS: Fitzpatrick skin phototypes proved to be an efficient method to study keloid and hypertrophic scars.
Keywords: Keloid. Cicatrix, hypertrophic. Skin pigmentation. Melanocytes. Ultraviolet rays.
RESUMO
INTRODUÇÃO: Queloide e cicatriz hipertrófica são cicatrizes patológicas com natureza fisiopatogênica comum, denominadas, em conjunto, cicatrizes fibroproliferativas. São mais frequentes em indivíduos de pele mais escura. Contudo, a atual miscigenação dificulta o enquadramento dos pacientes com variadas tonalidades de pele em classificações morfológicas e estáticas (branco ou caucasoide, mulato, pardo, hispânico ou latino, amarelo ou oriental ou mongoloide e negro ou negroide), e diferentes quanto à exposição solar. Sabe-se que pessoas oriundas de países de clima temperado ou frio quando residem em países tropicais aumentam a incidência dessas cicatrizes, principalmente nas áreas de maior exposição solar. Uma relação entre as cicatrizes fibroproliferativas e os fototipos de Fitzpatrick, classificação dinâmica baseada no relato do paciente quanto a sua resposta cutânea após a exposição solar, poderia contribuir para a compreensão da fisiopatologia dessas cicatrizes. Este estudo teve como objetivo investigar a distribuição das cicatrizes fibroproliferativas segundo os fototipos de Fitzpatrick.
MÉTODO: Foram avaliados 146 pacientes provenientes do Ambulatório da Disciplina de Cirurgia Plástica da Universidade Federal de São Paulo (Unifesp, São Paulo, SP, Brasil), portadores de qualquer tipo de cicatriz fibroproliferativa, em um ou mais locais do corpo. As cicatrizes fibroproliferativas dos pacientes foram classificadas de acordo com os critérios de Muir em cicatriz tipo queloide (Long-term Evolution, LTE), cicatriz tipo hipertrófica (Short-term Evolution, STE) e cicatriz tipo mista (Intermediate Group, IG), e os tipos de pele foram classificados segundo os fototipos de Fitzpatrick.
RESULTADOS: O fototipo Fitzpatrick III e a cicatriz mista foram mais frequentes entre os pacientes avaliados (P = 0,001). Houve associação (P = 0,025) entre as cicatrizes fibroproliferativas e os fototipos de Fitzpatrick, ou seja, quanto maior o fototipo maior a tendência de desenvolvimento de cicatrizes dos tipos queloide e mista.
CONCLUSÕES: Os fototipos de pele segundo Fitzpatrick mostraram-se válidos como critério a ser utilizado em estudos de queloide e cicatriz hipertrófica.
Palavras-chave: Queloide. Cicatriz hipertrófica. Pigmentação da pele. Melanócitos. Raio ultravioleta.
A keloid is a thick, raised scar that develops only in humans and extends laterally beyond the initial margins of the lesion1. It is primarily characterized by overproduction of collagen fibers and secondarily by fibroblast hyperplasia2,3. Keloid scars display variable color and continuous or recurrent growth. They do not show spontaneous regression and tend to regenerate upon surgical removal. Hypertrophic scars may often be confused with keloid scars. However, a hypertrophic scar does not cross the direction of the initial wound, regresses spontaneously, and has a better prognosis upon resection4.
There is a growing consensus toward considering keloid and hypertrophic scars as different intensities of phenotypic expression of the same fibropathogenic disorder. Thus, they are defined as fibroproliferative scars5-7. Because of the difficulty in some patients in classifying fibroproliferative scars based on their morphology, Muir6 defined them according to their prognosis. Three categories have been identified: the Short-Term Evolution scar (STE, or hypertrophic scar) that clinically corresponds to the hypertrophic scar, which is flatter and has a better prognosis; the Long-Term Evolution scar (LTE, or keloid scar) that corresponds to the keloid, which is nodular and has a worse prognosis; and the Intermediate Group scar (IG, or mixed scar), represented by keloids of the deltoid and scapular regions, which are flat and have a worse prognosis, and by ear keloids, which are nodular and have a better prognosis (Figure 1).
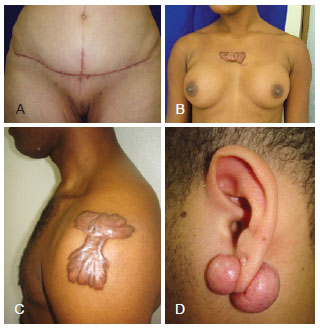
Figure 1 - Fibroproliferative scars. In A, hypertrophic scar (STE = Short-Term Evolution). In B, keloid scar (LTE = Long-Term Evolution). In C and D, mixed scar (IG = Intermediate Group).
A correlation between sun exposure and development of fibroproliferative scars has been observed. It seems that these pathological scars are prevalent in tropical countries3. When individuals whose ethnic origin is in colder countries move to tropical countries, they exhibit a higher incidence of these types of scars, mainly in parts of the body that are more exposed to the sun8.
Keloid and hypertrophic scars are common in individuals of darker skin3. However, mixing of "races" makes it difficult to group patients with different skin tones according to morphological and static classifications. Therefore, although no consensus on which classification should be used for these studies has been reached so far, several definitions for the different skin tones have been used, such as white for Caucasians; brown for biracial and multiracial individuals, and individuals of Spanish descent (Hispanic/ Latino); yellow for individuals of East Asian descent; and black for individuals of African descent, besides others of regional interpretation. However, the classification of Fitzpatrick skin phototypes9 takes into consideration phenotypic features and information reported by the patient, with regard to the effects caused by sun exposure on his or her skin. This would make it possible to include functional and dynamic aspects in the study. Hence, a correlation between fibroproliferative scars and Fitzpatrick skin phototypes would be more reliable than arbitrary "racial" categorization in the study of the frequency of these scars and would contribute to a better understanding of the pathophysiology of fibroproliferative scars.
Therefore, in this study, we aimed to investigate the distribution of keloid and hypertrophic scars according to Fitzpatrick phototypes.
METHOD
We analyzed 146 patients at the Outpatient Plastic Surgery Clinic of the Universidade Federal de São Paulo, Escola Paulista de Medicina (Federal University of São Paulo, Paulista School of Medicine) - (Unifesp-EPM, São Paulo, SP, Brazil) from January 2008 to January 2009. These patients presented with any type of fibroproliferative scar in one or more locations.
The age of the patients participating in the study varied from 15 to 60 years old (mean, 29 years; female, 61%; male, 39%). The average duration of the scars was 4.5 years, and ranged from 6 months to 45 years.
Patients who had undergone any prior treatment for lesions, such as those with burn scars and scars close to other scars, were not included in the study.
Fibroproliferative scars were classified according to the Muir criteria6 as STE or hypertrophic scars, LTE or keloids, and IG or mixed. Skin phototype was determined according to the classification obtained in Fitzpatrick's survey9 and was divided into 6 categories (I to VI).
Statistical analysis was performed with the Chi-square test; p < 0.05 was considered significant.
The study was approved by the Research Ethics Committee of UNIFESP. All the participants signed the Informed Consent Form.
RESULTS
Fitzpatrick phototype III was most common among patients with fibroproliferative scars (p = 0.001) (Figure 2). Mixed scars were commonly observed in these patients (p = 0.001) (Figure 3).
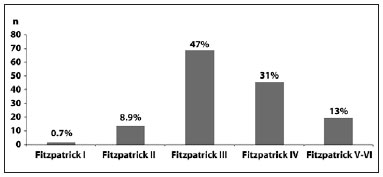
Figure 2 - Fitzpatrick phototype frequency in patients with fibroproliferative scars.
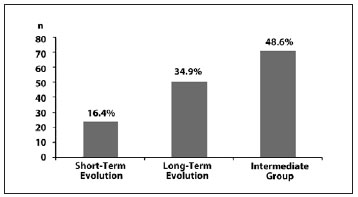
Figure 3 - Frequency of fibroproliferative scars according to the Muir classification.
A correlation (p = 0.025) between fibroproliferative scar types and Fitzpatrick phototypes (Table 1) was observed; the higher the Fitzpatrick phototype, the higher the tendency to develop keloid and mixed scars.
DISCUSSION
Keloid scars, most commonly observed in individuals of African, Spanish (Hispanic/Latino), or East Asian descent, have been associated with the levels of melanocytes, melanin, or alpha-melanocyte stimulating hormone (α-MSH)3.
Melanocytes produce and release the melanogenic neuropeptides α-MSH and corticotropin (adrenocorticotropic hormone, ACTH), known as "stress hormones." Moreover, melanocytes secrete and express catecholamine receptors for L-dihydroxyphenylalanine (L-DOPA) and serotonin10,11. In the presence of endogenous stress (e.g., inflammatory disease) and environmental stimuli (e.g., ultraviolet rays), melanocytes act as "stress" sensors in the epidermis12. Along with epidermal nerve fibers, melanocytes can function as the "cutaneous nervous system"13-15. This system acts mainly in the skin, controlling inflammation, immunity, functional regulation of cutaneous structures, thermoregulation, homeostasis modulation, and wound healing11,16. Thus, the aggressive exposure of melanocytes to sun radiation, which affects the cutaneous nervous system, might cause pathological skin disorders, also impairing wound healing15,17. This observation strengthens the possible correlation between keloid scars and cutaneous nervous system, since nerve fibers and melanocytes have the same neuroectodermal origin1.
Hypertrophic scars show a higher density of nerve fibers when compared to normotrophic scars14,18. Similarly, it has also been shown that keloids have the highest density of nerve fibers in the dermis of the skin, and are found in deeper areas17.
In addition, skin pigmentation influences cutaneous thermoregulation. In individuals of African descent, approximately 85% of the visible light spectrum is transformed into heat, whereas in Caucasians this percentage is 55%11,15. Therefore, natural or acquired variations in the constitutive pigmentation might vary the physiology of the skin and consequently, the healing process15,16. However, these data are not applicable to individuals of intermediate skin tones, thus justifying the necessity for new criteria to study the effect of sun exposure on the skin of all people in this group. This was the basis for the application of Fitzpatrick phototypes as part of the study design.
In the literature, data are still rare and inconclusive with regard to the possible implications of neuromelanogenic factors on keloid formation. The results obtained here indicate a correlation between fibroproliferative scars and skin phototypes. In the patients analyzed in this study, we observed that hypertrophic scar was less common; however, this information is not supported in clinical practice, where hypertrophic scars are more prevalent. This result might be explained with the minor psychological and social relevance of hypertrophic scarring. As this type of pathologic scar shows a milder phenotype and better prognosis19 because of its self-limiting tendency and spontaneous regression, most patients do not seek the help of specialized health services such as outpatient clinics20.
On the other hand, it was observed that mixed scars were the most frequent in this study. This data strengthens the correlation between fibroproliferative scars and the body parts that are more exposed to ultraviolet rays, particularly in tropical countries. Further studies are required to elucidate new methods of fibroproliferative scar prevention that take into consideration the correlation between ultraviolet radiation, melanogenesis, and wound healing.
CONCLUSIONS
Fitzpatrick skin phototypes have been shown to be an efficient classification that should be considered for studies on keloid and hypertrophic scars.
REFERENCES
1. Hochman B, Vilas Bôas FC, Mariano M, Ferreira LM. Keloid heterograft in the hamster (Mesocricetus auratus) cheek pouch, Brazil. Acta Cir Bras. 2005;20(3):200-12.
2. Olabanji JK, Onayemi O, Olasode OA, Lawai OAR. Keloids: an old problem still searching for a solution. Surg Practice. 2005;9:2-7.
3. O'Sullivan ST, O'Shaughnessy M, O'Connor TP. Aetiology and management of hypertrophic scars and keloids. Ann R Coll Surg Engl. 1996;78(3 Pt 1):168-75.
4. Al-Attar A, Mess S, Thomassen JM, Kauffman CL, Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117(1):286-300.
5. Köse O, Waseem A. Keloids and hypertrophic scars: are they two different sides of the same coin? Dermatol Surg. 2008;34(3):336-46.
6. Muir IF. On the nature of keloid and hypertrophic scars. Br J Plast Surg. 1990;43(1):61-9.
7. Rahban SR, Garner WL. Fibroproliferative scars. Clin Plast Surg. 2003;30(1):77-89.
8. Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989;84(5):827-37.
9. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869-71.
10. Misery L. The neuro-immuno-cutaneous system and ultraviolet radiation. Photodermatol Photoimmunol Photomed. 2000;16(2):78-81.
11. Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155-228.
12. Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71(4):493-500.
13. Ferreira LM, Gragnani A, Furtado F, Hochman B. Control of the skin scarring response. An Acad Bras Cienc. 2009;81(3):623-9.
14. Parkhouse N, Crowe R, McGrouther DA, Burnstock G. Painful hypertrophic scarring and neuropeptides. Lancet. 1992;340(8832):1410.
15. Slominski A, Paus R, Schadendorf D. Melanocytes as "sensory" and regulatory cells in the epidermis. J Theor Biol. 1993;164(1):103-20.
16. Besné I, Descombes C, Breton L. Effect of age and anatomical site on density of sensory innervation in human epidermis. Arch Dermatol. 2002;138(11):1445-50.
17. Hochman B, Nahas FX, Sobral CS, Arias V, Locali RF, Juliano Y, et al. Nerve fibres: a possible role in keloid pathogenesis. Br J Dermatol. 2008;158(3):651-2.
18. Crowe R, Parkhouse N, McGrouther D, Burnstock G. Neuropeptide-containing nerves in painful hypertrophic human scar tissue. Br J Dermatol. 1994;130(4):444-52.
19. Furtado F, Hochman B, Ferrara SF, Dini GM, Nunes JM, Juliano Y, et al. What factors affect the quality of life of patients with keloids? Rev Assoc Med Bras. 2009;55(6):700-4.
20. Furtado F, Hochman B, Farber PL, Muller MC, Hayashi LF, Ferreira LM. Psychological stress as a risk factor for postoperative keloid recurrence. J Psychosom Res. 2012;72(4):282-7.
1. Physician; Full member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society for Plastic Surgery) - SBCP; Affiliate Professor in the Plastic Surgery Division at the Universidade Federal de São Paulo, Escola Paulista de Medicina (Federal University of São Paulo, Paulista School of Medicine) - Unifesp-EPM, São Paulo, SP, Brazil.
2. Undergraduate medical student at Unifesp-EPM, São Paulo, SP, Brazil.
3. Master's degree in Plastic Surgery of Unifesp-EPM; Physiotherapist, São Paulo, SP, Brazil.
4. Doctor in Plastic Surgery of Unifesp-EPM, Assistant Professor in the Postgraduate program in Plastic Surgery at Unifesp-EPM, São Paulo, SP, Brazil.
5. Full professor of the Plastic Surgery Division at Unifesp-EPM; Full member of SBCP; Coordinator of Medicine III at Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordination for the Improvement of Higher Education Personnel) - CAPES; Scientist 1B at the Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development) - CNPq, São Paulo, SP, Brazil.
Correspondence to:
Bernardo Hochman
Plastic Surgery Division - Federal University of São Paulo
Rua Napoleão de Barros, 715 - 4o andar
São Paulo, SP, Brazil - CEP 04024-002
E-mail: bernardohochman@uol.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: January 22, 2012
Article accepted: May 15, 2012
This study was performed at the Universidade Federal de São Paulo, Escola Paulista de Medicina (Federal University of São Paulo, Paulista School of Medicine) - Unifesp-EPM, São Paulo, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter