

Original Article - Year 2012 - Volume 27 -
A new animal model for training rhinoplasty
Novo modelo animal para treinamento de rinoplastia
ABSTRACT
BACKGROUND: Rhinoplasty poses a unique set of challenges to the plastic surgeon. Surgical dexterity is associated with a long learning curve; this sometimes causes undesirable results, especially in the case of surgeons who are beginners at performing such a complex procedure. Understanding the anatomic basis and mechanical properties of its components is fundamental for achieving satisfactory results in rhinoplasty. To enhance surgical training for those learning the principles of rhinoplasty, a wide variety of models has been described. Among these are the nose of the pig and rabbit, chicken sternal cartilage, nasal silicone models, and even a virtual computer-generated 3-dimensional model of the human nose.
OBJECTIVE: The authors propose the use of a sheep head as a model to replicate the technical steps involved in rhinoseptoplasty for surgical education and research.
METHODS: Several fresh domestic sheep heads were obtained from a local butcher shop, dissected according to a predetermined set of steps, and modeled in different shapes as described in the literature for human models. Sutures were placed in the cartilage to simulate the steps of a structured rhinoplasty.
RESULTS: The caprine model presents anatomical structures similar to those found in humans, including the medial and lateral crura, with appropriate cephalic orientation and domal angles. Septal cartilage was sufficient to simulate several grafts, and bones were available for fracture and rasping.
CONCLUSIONS: With an estimated more than 1 billion domestic sheep worldwide, this model has the potential to improve outcomes in rhinoplasty by providing wider opportunities for training in a procedure that requires knowledge, precision, and artistry.
Keywords: Rhinoplasty. Nose/surgery. Plastic surgery. Training.
RESUMO
INTRODUÇÃO: A rinoplastia apresenta um conjunto único de desafios para o cirurgião plástico. Destreza cirúrgica exige longa curva de aprendizado, que pode trazer resultados, por vezes, problemáticos, especialmente para o iniciante em um procedimento tão complexo. Entender as bases anatômicas e as propriedades mecânicas dos componentes nasais é fundamental para obter resultados satisfatórios na rinoplastia. Para melhorar o treinamento do cirurgião iniciante em rinoplastia, grande variedade de modelos tem sido descrita, como nariz de porco ou coelho, cartilagem do esterno de frango, modelos de silicone e, até mesmo, modelo virtual tridimensional do nariz humano gerado por computador. Neste artigo é proposto o uso da cabeça de ovelha como modelo para reprodução dos passos técnicos da rinosseptoplastia, constituindo uma ferramenta para educação e pesquisa cirúrgica.
MÉTODO: Várias cabeças de ovelha foram dissecadas de forma reprodutível e as cartilagens nasais foram modeladas em diferentes formas, tal como descrito na literatura para modelo humano. Suturas foram colocadas nas cartilagens para simular os passos da rinoplastia estruturada.
RESULTADOS: O modelo de caprinos reproduziu estruturas similares, incluindo as cruras medial e lateral e os ângulos domais, além de proporcionar orientação cefálica apropriada. A cartilagem septal é abundante para simulação de enxertos e vários ossos estão disponíveis para fratura e raspagem.
CONCLUSÕES: Com um número estimado de mais de um bilhão de ovelhas domésticas no mundo, esse modelo tem o potencial de melhorar os resultados na rinoplastia, proporcionando maior oportunidade de treinamento em um procedimento que requer precisão, conhecimento e arte.
Palavras-chave: Rinoplastia. Nariz/cirurgia. Cirurgia plástica. Capacitação.
Rhinoplasty poses a unique set of challenges to the plastic surgeon; even with the currently available knowledge and technology, undesirable results can be obtained even by experts. Rhinoplasty is different from other kinds of aesthetic surgery in that a single millimeter may be the difference between perfection and an undesirable result. The nose presents additional challenges to the surgeon because function must always be addressed in association with the aesthetic problem and because the healing process can distort the achieved perfect surgical result1-3.
Traditionally, rhinoplasty either for aesthetic or functional purposes is often performed simultaneously with septoplasty and partial inferior turbinectomy. These procedures decrease nasal resistance, thereby treating chronic nasal airway obstruction, which is refractory to other, more conservative, methods of treatment1,4.
Dexterity in performing rhinoplasty is associated with a long learning curve; this can sometimes cause undesirable results, especially for a surgeon who is a beginner at this complex procedure.
Understanding the anatomic basis and mechanical properties of its components is fundamental in achieving satisfactory results in rhinoplasty. Surgical skill is expected to develop only with considerable experience5.
After residency training, many new surgeons begin practicing privately and, for various reasons, distance themselves from academia that could provide opportunities for constant learning and improvement.
To enhance educational training for beginners and for research on the principles of rhinology, a wide variety of models has been described. Among these are goats6, pigs7, mice8, and lambs9.
Rhinoplasty frequently includes modeling cartilages, and chicken sternal cartilage has been proposed as an educational model for improving carving ability10.
The authors propose the use of a sheep head as a model to replicate the technical steps involved in aesthetic rhinoplasty for surgical education and research.
METHOD
An extensive search on PubMed and SciELO was performed using the keywords "plastic surgery sheep", "animal model lambs", "animal models rhinoplasty", "animal models plastic surgery", and "rhinoplasty models" to identify previous studies on the sheep model. We did not find any such studies. Therefore, we obtained several fresh domestic sheep heads from a local butcher shop and preserved them in a freezer. Before the dissection of each model, the frozen head was kept in a sink for 10 minutes with water at 68-77°F running over its nose. The head was then superficially dried with paper towels (kitchen roll) and placed in a plastic foodstorage container.
Nasal cartilages were dissected by following a predetermined set of steps and were modeled in various shapes as described in the literature for human models. Sutures were placed in the cartilages to simulate the steps of a structured rhinoplasty.
Operative Technique
First, the area to be dissected (the area under the skin of the nose and the septal mucosa) was infiltrated with 0.9% saline to promote hydrodissection.
A V-shaped incision was made with a Nº 15 scalpel blade in the middle of the skin of the columella, extending to the cutaneous margin of the upper lateral crura. We then lifted the skin flap of the nasal dorsum at the level closest to the cartilage using a curved and delicate scissor. The flap presented with a delicate double hook. After this, we performed a precise anatomical dissection of the nasal cartilages and bones of the model, as is normally done in anatomic dissections of the human nose, to identify similarities and differences between humans and the animal model (Figure 1).
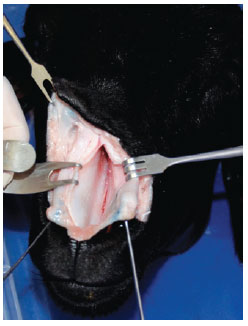
Figure 1 - Exposing the septal cartilage.
RESULTS
The anatomical structures of the caprine model are similar to those of humans, including the medial and lateral crura, and have appropriate cephalic orientation and domal angles. The anterior-posterior dimension of the lateral crura was 8 millimeters (0.31 inches) with a cephalocaudal length of 12 mm (0.47 inches). The lateral crural thickness was approximately 1 mm (0.04 inches). The average angle of divergence was 70°. The average interdomal distance was 13 mm (0.51 inches), and the average domal width was 6 mm (0.24 inches).
After separating the alar cartilages, the medial crura of the sheep were found to be much thinner and more difficult to dissect than those of humans (Figure 2).
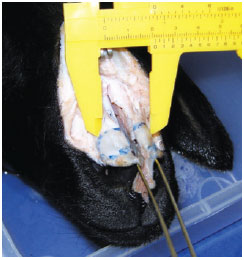
Figure 2 - Separating the medial crura and exposing the caudal septum.
The interdomal suspensory ligament was incised to expose the anterior septal angle. The perichondrium was then incised with a No. 15 scalpel, and a Cottle elevator was placed to create bilateral superior tunnels. In the subperichondrial plane, the elevation was extended posteriorly along the crest in the posterior direction at the level of the septal angle. The upper lateral cartilages were disconnected from the nasal septal cartilage (which coincides with the junction of the nasal valve angle). The cartilage graft was designed and removed from the septum, leaving a 1-cm (0.4 inches) wide dorsal-caudal L-shaped strut (Figure 3), and a large piece of the donor cartilage septum was removed.
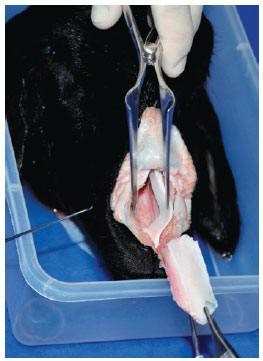
Figure 3 - Cutting the septum, leaving a 1-centimeter wide L-shaped strut.
The cartilaginous hump, the caudal portion of the septum, and the quadrangular donor graft were removed with a No 15 blade. All this material was available for training in onlay, radix, cap, subdomal, lateral crural spanning, spreader, alar contour, or strut grafts.
The spreader grafts were placed at the "L" structure in the septal cartilage and sutured with the nonabsorbable mononylon 4-0 suture (Figure 4).

Figure 4 - Placement of spreader grafts.
The cephalic portion of the alar cartilages was resected after the perichondrium and connective tissues on the lateral cartilage were detached in all cases.
Transdomal and interdomal sutures were placed with 4-0 nonabsorbable sutures (Figure 5), as well as fixing sutures, in the upper lateral cartilage in the spreaders and quadrangular septal cartilage. Fixing sutures were placed in the medial crura in the caudal septum and then removed, to study the gain in projection and alterations in the tripod concept.

Figure 5 - Transdomal and interdomal sutures being placed.
The tissue between the medial crura and the columella was sharply dissected, and a columellar strut graft was placed.
A variety of tip grafts (extended columellar strut tip, onlay, shield, and subdomal) were also placed (Figure 6). A turndown cephalic lateral crural graft was unsuccessful.
Figure 6 - Practicing the placement of a tip graft.
Any desired nasal osteotomy can be performed, as in this example of a "J" low-to-high approach in the ascending maxilla (Figure 7). Additional osteotomy (with the external osteotome) can be practiced in the long, large nasal bone present in this animal model.
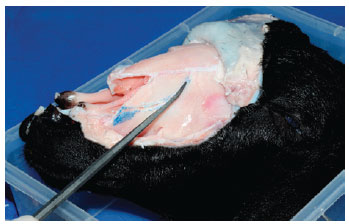
Figure 7 - Fracture of the ascending maxilla, preserving an imagined "Webster" triangle.
The ascending process of the maxilla is 0.9-mm thick in the fracture line (Figure 8).
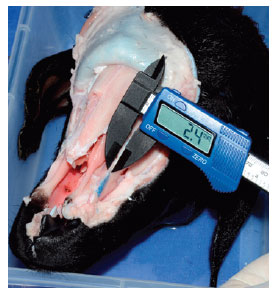
Figure 8 - Measurement of the thickness of the ascending process of the maxilla
After removing the fractured bones, an additional 1.96 inches of septal cartilage was found.
Inferior turbinectomy could be performed easily, and the middle and superior turbinates were also at full range, as were all the cranial sinuses.
Bone rasping was performed a manner similar to that performed in rhinoplasties (Figure 9). An additional fracture can be made with an external osteotome as a form of additional training.
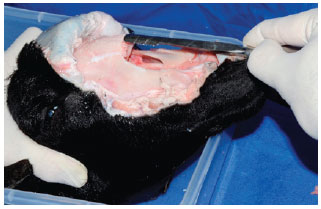
Figure 9 - Dorsal nasal bone rasping.
DISCUSSION
Rhinoplasty for both functional and aesthetic purposes, has become one of the most frequently requested and performed surgical procedures.
The nose is located halfway between the eyes and mouth. Any abnormal feature in the nose is immediately noticeable. Nasal deviation is a challenge to the surgeon, because it is usually associated with functional problems.
For these reasons, postrhinoplasty complications are regarded as challenging problems for both the patient and the surgeon11. Some cases necessitate grafts for better aesthetic or functional outcome. The use of autogenous grafts in the nose can be quite challenging.
Of the commonly used autogenous materials for correction of nasal deviation, septal cartilage is the ideal source material because it is of good quality, is flexible, and is easy to obtain. When there is a shortage of this cartilage, auricular or costal cartilage may be an option, depending on the surgeon's preference and ability.
Carving septal cartilage into grafts is a difficult process. Precision and results improve with clinical experience on human patients, but the sheep experimental model is useful for training surgeons to perform rhinoplasties. The nasal anatomy of the sheep was established in detail to provide a guide for researchers with regard to its use as a convenient experimental model for rhinoplasty, with all stages similar to those performed for humans.
The anatomy of the nose and nasal cavities of the sheep can constitute a guide for researchers as a convenient experimental model for any kind of proposed correction of septal deviations as well as for training of several modalities of turbinate and sinus procedures. The stages involved are similar to those involved when working on humans, and this model is associated with a very low cost and the possibility of surgical simulation even at home.
The skin of the sheep's nose is so thick that surgical maneuvers for treating rhinophyma can be practiced12.
CONCLUSIONS
With an estimated more than 1 billion domestic sheep worldwide, this model has the potential to improve outcomes in rhinoplasty, providing greater opportunities for training in a procedure that requires knowledge, precision, and artistry.
REFERENCES
1. Modolin M, Baracat GZ, Kamakura L, Cintra W Jr, Cruz LG, Ferreira MC. Histological comparison of the alar nasal cartilages in unilateral cleft lip. Rev Hosp Clin Fac Med São Paulo. 2002;57(4):143-6.
2. Dini GM, Ferreira LM. Rhinoplasty and PubMed. Plast Reconstr Surg. 2006;118(1):289.
3. Dini GM, Albuquerque LG, Ferreira LM. The future of rhinoplasty and the Dallas rhinoplasty symposium. Plast Reconstr Surg. 2009;123(2):64e-65e.
4. Arantes HL, Silva RFP, Mélega JM. Simetria nasal após a realização de rinoplastia associada à queiloplastia em crianças com fissura labial e labiopalatal. Rev Bras Cir Plást. 2011;26(1):48-53.
5. Gunter JP, Rohrich RJ, Adams Jr WP. Dallas rhinoplasty: nasal surgery by the masters. 2nd ed. St Louis: Quality Medical Publishing; 2007.
6. Ford RL, Barsam A, Velusami P, Ellis H. Drainage of the maxillary sinus: a comparative anatomy study in humans and goats. J Otolaryngol Head Neck Surg. 2011;40(1):70-4.
7. Stelzle F, Benner KU. An animal model for sinus floor elevation with great elevation heights. Macroscopic, microscopic, radiological and micro-CT analysis: ex vivo. Clin Oral Implants Res. 2010;21(12):1370-8.
8. Lindsay R, Slaughter T, Britton-Webb J, Mog SR, Conran R, Tadros M, et al. Development of a murine model of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2006;134(5):724-30.
9. Mladina R, Vuković K, Stern Padovan R, Skitarelić N. An animal model for endoscopic endonasal surgery and dacryocystorhinostomy training: uses and limitations of the lamb's head. J Laryngol Otol. 2011;125(7):696-700.
10. Weinfeld AB. Chicken sternal cartilage for simulated septal cartilage graft carving: a rhinoplasty educational model. Aesthet Surg J. 2010;30(6):810-3.
11. Sperli AE, Freitas JOG, Fischler R. Tratamento cirúrgico da columela retraída em narizes primários. Rev Bras Cir Plást. 2011;26(4):613-7.
12. Costa TC, Firme WAA, Brito LMR, Vieira MBG, Leite LAS. Rinofima: opções cirúrgicas utilizadas no Serviço de Cirurgia Plástica do Hospital Agamenon Magalhães - PE. Rev Bras Cir Plást. 2010;25(4):633-6.
1. Doctor in Plastic Surgery, professor in Plastic Surgery at Pontifícia Universidade Católica de São Paulo (Pontifical Catholic University of São Paulo) - PUC, Sorocaba, SP, Brazil.
2. Doctor in Plastic Surgery, full professor in Plastic Surgery at PUC, Sorocaba, SP, Brazil.
3. Resident in Plastic Surgery at PUC, Sorocaba, SP, Brazil.
Correspondence to:
Gal Moreira Dini
Rua Vicencia Faria Versage, 318
Sorocaba, SP, Brazil - CEP 18031-080
E-mail: dr.gal@uol.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: February 5, 2012
Article accepted: April 22, 2012
Work conducted at the Plastic Surgery Department of the Pontifícia Universidade Católica de São Paulo (Pontifical Catholic University of São Paulo) - PUC, Sorocaba, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter