

Original Article - Year 2012 - Volume 27 -
Bioplasty for lipodystrophy in patients with HIV/AIDS
Bioplastia na lipodistrofia de pacientes com HIV/AIDS
ABSTRACT
BACKGROUND: When the treatment of acquired immunodeficiency syndrome (AIDS) with highly active antiretroviral therapy (HAART) began in the 1990s, it considerably increased the life expectancy and quality of life of AIDS patients. However, the decrease in morbidity and mortality associated with opportunistic infectious and neoplastic diseases was accompanied by an increase in the prevalence of other diseases, including HIV-associated lipodystrophy. Lipodystrophy is due to the toxicity of drugs used in antiretroviral therapy, including protease inhibitors and nucleoside analog reverse transcriptase inhibitors. This article discusses the treatment of facial lipodystrophy, which confers an appearance of premature aging and brings back the old stigma of the "AIDS face," which negatively impacts the quality of life of HIV carriers.
METHODS: Forty-one patients with facial lipoatrophy received filling with polymethylmethacrylate (PMMA) at the Hospital Universitário da Universidade Federal de Juiz de Fora (HU-UFJF) and at the Plastic Center Clinic, Plastic Surgery Clinic in Juiz de Fora between January 2010 and February 2012.
RESULTS: Patients received 1 to 4 procedures with a minimum interval of 90 days between procedures. The amount of PMMA used ranged from 3 to 18 mL per procedure according to the degree and region to be corrected. The results were aesthetically favorable in all patients.
CONCLUSIONS: The results obtained through bioplasty with PMMA are considered satisfactory by patients. The material used is highly adaptable to the receiving areas, requiring only modeling and an adequate amount in order to obtain good aesthetic results.
Keywords: HIV-associated lipodystrophy syndrome. Acquired immunodeficiency syndrome. HIV. Lipodystrophy.
RESUMO
INTRODUÇÃO: O início do tratamento da síndrome da imunodeficiência adquirida (AIDS) com a terapia antirretroviral de alta atividade (HAART), na década de 1990, aumentou, consideravelmente, a longevidade e a qualidade de vida dos portadores da doença. A redução da morbidade e da mortalidade associadas a doenças infecciosas e neoplásicas oportunistas, porém, tem sido acompanhada pelo aumento da prevalência de outras doenças, entre elas a lipodistrofia associada ao vírus da imunodeficiência humana (HIV). A lipodistrofia decorre da toxicidade de drogas utilizadas na terapia antirretroviral, sendo atribuída aos inibidores de protease e aos inibidores da transcriptase reversa análogos do nucleosídeo. Este trabalho aborda a lipoatrofia facial, que confere um aspecto de envelhecimento precoce e traz de volta o velho estigma da "facies da AIDS", podendo impactar negativamente na qualidade de vida dos portadores de HIV.
MÉTODO: Neste estudo foram incluídos 41 pacientes apresentando lipoatrofia facial, que foram submetidos a preenchimento com polimetilmetacrilato (PMMA) no Hospital Universitário da Universidade Federal de Juiz de Fora (HU-UFJF) e na clínica Plastic Center, Clínica de Cirurgia Plástica em Juiz de Fora, no período entre janeiro de 2010 e fevereiro de 2012.
RESULTADOS: O número de procedimentos realizados em cada paciente variou de 1 a 4, sendo respeitado um intervalo mínimo de 90 dias entre eles. A quantidade de PMMA utilizado variou de acordo com o grau e a região a serem corrigidos, ficando entre 3 ml e 18 ml por procedimento. Em todos os pacientes, o resultado obtido foi favorável esteticamente.
CONCLUSÕES: Os resultados obtidos pela bioplastia com PMMA foram considerados satisfatórios pelos pacientes. O material utilizado possui alta adaptabilidade às áreas receptoras, necessitando apenas da modelagem e da quantidade adequada para que apresente bom padrão estético.
Palavras-chave: Síndrome de lipodistrofia associada ao HIV. Síndrome de imunodeficiência adquirida. HIV. Lipodistrofia.
When the treatment of acquired immunodeficiency syndrome (AIDS) with highly active antiretroviral therapy (HAART) began in the 1990s, it considerably increased patients' life expectancy and quality of life. However, the decrease in morbidity and mortality due to opportunistic infections and neoplastic diseases has been accompanied by an increase in the prevalence of other diseases, including human immunodeficiency virus (HIV)-associated lipodystrophy1.
The morphological signs of lipodystrophy were described about 2 years after protease inhibitors (PIs) were introduced and were initially attributed to their toxicity. "Crixbelly", the name given to the changes caused by the use of Crixivan® (Indinavir), a protease inhibitor, was the first designation2 for such changes in 1998. However, PIs were introduced simultaneously with a second nucleoside analog reverse-transcriptase inhibitor (nRTI), stavudine3, which also induced similar metabolic changes, leading to that name falling out of use4.
The physiopathological mechanism of the syndrome caused by the use of such medicines has not been completely clarified5. PIs are believed to inhibit adipocyte proliferation and differentiation, and increase lipolysis via the inhibition of SREBP-1, which blocks the activation of transcription factors bound to PPAR-gamma. On the other hand, nRTIs (especially stavudine) may be related to the induction of mitochondrial dysfunction, which leads to lipoatrophy. Disturbances in fatty acid transport that predispose patients to centripetal fat accumulation have also been described1. Therefore, new supplementary studies are necessary to better understand this mechanism.
The main feature of this syndrome is poor body fat distribution, both internal and external. There is loss of fat in the face, glutei, legs, and arms and fat accumulation in the abdomen, back, posterior cervical region, and breasts.
This dysfunctional distribution is termed HIV lipodystrophic syndrome (HIVLS). In HIVLS, besides an abnormal redistribution of body fat, other changes such as changes in glucose metabolism, insulin resistance, and dyslipidemia may occur2,4; such changes may appear in combination or isolation There are reports in which the patient only exhibits a loss or gain of fat in one of those regions, which allows HIVLS to be classified into 3 categories2,6:
Lipoatrophy is characterized by a decrease in body fat in the peripheral regions such as the arms, legs, face, and buttocks; it may present relative muscular and venous prominences; Lipohypertrophy is characterized by the accumulation of fat in the abdominal region, the presence of dorsal gibbosity, gynecomastia, and increased breast volume in women; The mixed form is characterized by a combination of components of the 2 previously described forms.
Patients receiving antiretroviral therapy must be cognizant of changes to their body. Moreover, they must regularly consult the physician responsible for their clinical follow-up. Treatment of these physical changes requires individualized care. Although no specific consensus exists, several options are discussed in the literature7.
The first measure healthcare professionals can adopt besides giving special attention to food and the practice of physical activities is to consider changing the class of the drug the patient is currently taking. These 2 actions may limit the damage caused by lipodystrophy and even prevent the symptoms from appearing. Metformin, growth hormone, or modern antiretroviral drugs are some available alternatives2.
More serious changes may be corrected with reparative procedures through plastic surgery. Although palliative in nature, they are available at several institutions of the public health network nationwide. Some of the most frequent are facial filling with polymethylmethacrylate (PMMA) for the treatment of facial lipoatrophy; liposuction in the abdomen, back, and posterior cervical region; and silicone prosthetic implants in the gluteus6.
This article discusses facial lipodystrophy (Figure 1), which confers an appearance of premature aging and brings back the old stigma of the "AIDS face", which can negatively impact the quality of life of HIV carriers. Such an appearance can cause the revelation of seropositivity, depression, isolation, social exclusion, poor compliance, and even abandonment of treatment3.
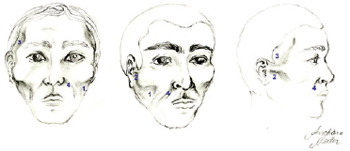
Figure 1 - Main areas of facial lipodystrophy: (1) malar region; (2) preauricular region; (3) temporal region; (4) nasogenian groove.
METHODS
In this study, 41 patients who underwent surgical procedures for the treatment of facial lipoatrophy at the Hospital Universitário da Universidade Federal de Juiz de Fora (HU-UFJF) and the Plastic Center Clinic, Plastic Surgery Clinic in Juiz de Fora, from January 2010 to February 2012 were included.
Facial lipoatrophy was diagnosed according to clinical evaluation including the presence and duration of signs and symptoms of the disease. Bioplasty was used to repair facial lipoatrophy, which involves the creation or reconstruction of the angles and contours lost as a result of the decrease in volume through fat resorption (i.e., lipoatrophy), cartilage loss, hypertrophic processes (i.e., subnormal development processes), hypotonia, and muscle stretching8.
PMMA is a polymer used as filler in the form of synthetic microspheres of 40-60 mm in diameter and carried in a suspension media that may be collagenic, aproteic, or crystalloid. Commercial forms may have PMMA concentrations of 2%, 5%, 10%, 15%, or 30%. The product is of a permanent character, with only the carrier being absorbed. It is used to fill ridges, deep wrinkles, scars, dermal defects, soft tissues, and bone tissues8.
Inclusion Criteria
Confirmed HIV infection; Facial lipoatrophy; Ability to understand and follow the instructions given by the medical team; Signing of informed consent form; Willingness to participate in study protocols; Signing of authorization form to have his/her pictures published in scientific journals.
Exclusion Criteria
T CD4+ cell count less than 200 cells/mL (test obtained within 120 days prior to the procedure); Viral load > 5,000; Presence of signs of bacterial or viral infection in any anatomical structure (e.g., face, oral cavity, upper respiratory tract, etc.); Unavailable to participate in study protocols; Absence in outpatient return visits scheduled by the plastic surgery team for follow-up.
Technique for Applying PMMA to the Face
Marking the area to be treated with indelible ink, with the patient in the orthostatic or sitting position; Local antisepsis with non-alcoholic chlorhexidine; Application of local anesthetic with xylocaine 2% and marcain 0.5%; Skin perforation with 1.2 × 40 mm needle (18G x 1 ½"); Introduction of 70 × 0.9 mm microcannula; Retrograde PMMA injection: the product is injected as the microcannula is removed; the patient must be in a sitting position with the head end of the bed at an angle of 45 or 60 degrees; Modeling with soft finger movements.
RESULTS
The number of procedures performed on each patient ranged from 1 to 4, with a minimum interval of 90 days between each procedure. The amount of PMMA used ranged from 3 to 18 mL per procedure according to the degree and region to be corrected.
Among the observed complications, local inflammation was treated with a combination of ice and anti-inflammatory drugs; nodules, which occurred in 5 patients, were treated with infiltration of triamcinolone 20 mg/mL. The number of applications of this substance varied according to the size of the lesions to be corrected.
The results obtained were aesthetically favorable in all patients (Figures 2 to 5).
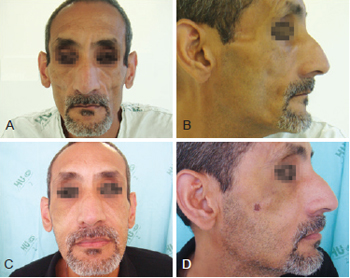
Figure 2 - Patient 1. In A and B, appearance prior to bioplastic treatment. In C and D, 2 months after bioplasty with PMMA.
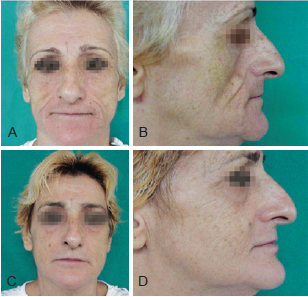
Figure 3 - Patient 2. In A and B, appearance prior to bioplastic treatment. In C and D, 6 months after bioplasty with PMMA.
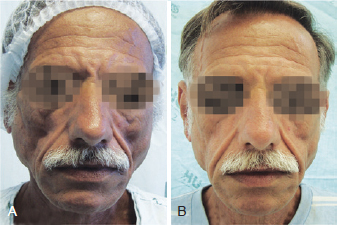
Figure 4 - Patient 3. In A, appearance prior to bioplastic treatment. In B, 6 months after bioplasty with PMMA.
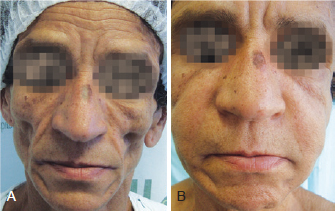
Figure 5 - Patient 4. In A, appearance prior to bioplastic treatment. In B, 2 months after bioplasty with PMMA.
DISCUSSION
Metabolic changes involving HIV infection and treatment and their classification have evolved as new study methods have emerged. The designation of this syndrome changed over time; today, it is designated as body fat redistribution syndrome, antiretroviral (ARV) therapy-associated metabolic syndrome, and HIV/HAART-associated dyslipidemic lipodystrophy (HADL)9.
It is extremely important to establish the link between lipodystrophy and the start of AIDS treatment using HAART, especially if it involves PIs and nRTIs. However, lipodystrophy can also be observed without drug therapy, suggesting that both have similar starting mechanisms6.
Lipodystrophy may be better understood in patients with prolonged drug use. However, early adverse effects may also be present. Moreover, the risk of those events varies by drug, drug class, and patient4.
There are also concerns about the risk of cardiovascular disease, one of the long-term effects of lipodystrophy7. This is because the predisposing factors for cardiovascular disease are the same as those for the changes caused by lipodystrophy, namely abnormal redistribution of body fat, changes in glucose metabolism, insulin resistance, and dyslipidemia.
In addition, many lipodystrophic patients are afraid of their seropositivity being exposed, which is an important source of concern and stress. This is because the signs of this syndrome are visible, especially on the face, making these patients vulnerable to their diagnosis being discovered by others, consequently resulting in prejudice and discrimination2.
Despite the advances in drugs that inhibit HIV replication, reducing viral load to values to less than 50 copies/mL and research methods that can detect such loads4, complications related to their use have become more common. Consequently, compliance with therapy becomes more problematic in patients reporting decreased quality of life after the medication is introduced.
An important concept for healthcare professionals working directly with HIV-positive patients receiving HAART therapy is the distinction between self-limiting adverse effects and potentially serious effects. Although no specific treatments or consensuses exist, suggestions concerning diet, lifestyle changes, and the use of hypolipidemic drugs or regimes that increase insulin sensitivity may be useful2,7. In addition, more complex procedures such as bioplasty may be useful.
As such, the treatment of facial lipodystrophy with PMMA, a theoretically palliative method, must not be regarded as an aesthetic procedure only; it should also be regarded as a technique aiming to decrease the stigma of AIDS and preventing the patient from having depressive thoughts, suffering prejudice and discrimination, and abandoning antiretroviral treatment.
The complexity and multidimensionality of lipodystrophy-related factors make the advancement of further research on this subject very important10.
Through the ministerial directive SAS/MS No. 118/05 (DOU, 2005), the Ministry of Health instituted the performance of surgical procedures with PMMA application in HIV-positive patients who have free and universal access to the National Health Service. This measure is intended to alleviate the negative repercussions that lipodystrophy can cause in those patients, such as negative body image perception, fear of forced revelation of their diagnosis, abandonment of antiretroviral therapy, and even social withdrawal.
Skin appearance, texture, turgor, and elasticity are considered to be natural as long as the technique used is performed well.
Because of PMMA's permanent nature, hypercorrection must be avoided; hypercorrection is sometimes required to emphasize the results when transient injectable material is used11.
CONCLUSIONS
The results obtained through bioplasty with PMMA are considered satisfactory by patients. The material used is highly adaptable to the receiving areas, requiring only modeling and an adequate amount in order for good aesthetic results to be obtained.
ACKNOWLEDGEMENTS
The authors wish to thank Andiara Barbosa Neder, Graduate of Arts and Design, Universidade Federal de Juiz de Fora (UFJF), for the illustration included in this article.
REFERENCES
1. Diehl LA, Dias JR, Paes ACS, Thomazini MC, Garcia LR, Cinagawa E, et al. Prevalência da lipodistrofia associada ao HIV em pacientes ambulatoriais brasileiros: relação com síndrome metabólica e fatores de risco cardiovascular. Arq Bras Endrocrinol Metab. 2008;52(4):658-67.
2. Valente AMM, Reis AF, Machado DM, Succi RCM, Chacra AR. Alterações metabólicas da síndrome lipodistrófica do HIV. Arq Bras Endocrinol Metab. 2005;49(6):871-81.
3. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de DST, Aids e Hepatites Virais. Manual de tratamento da lipoatrofia facial: recomendações para o preenchimento facial com polimetilmetacrilato em portadores de HIV/Aids. Brasília: Ministério da Saúde; 2009.
4. Montessori V, Press N, Harris M, Akagi L, Montaner JS. Adverse effects of antiretroviral therapy for HIV infection. CMAJ. 2004;170(2):229-38.
5. Thiébaut R, Daucourt V, Mercié P, Ekouévi DK, Malvy D, Morlat P, et al. Lipodystrophy, metabolic disorders, and human immunodeficiency virus infection: Aquitaine cohort, France, 1999. Groupe d'Epidémiologie Clinique du Syndrome d'Immunodéficience Acquise en Aquitaine. Clin Infect Dis. 2000;31(6):1482-7.
6. Disponível em: http://www.aids.gov.br/pagina/efeitos-colaterais. Acesso em: 20/4/2012.
7. Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48-62.
8. Vargas AF, Amorim NG, Pintaguy I. Complicações tardias dos preenchimentos permanentes. Rev Bras Cir Plást. 2009;24(1):71-81.
9. Balasubramanyan A, Sekhar RV, Jahoor F, Jones PH, Pownall HJ. Pathophysiology of dyslipidemia and increased cardiovascular risk in HIV lipodystrophy: a model of 'systemic steatosis'. Curr Opin Lipidol. 2004;15(1):59-67.
10. Seidl EMF, Machado ACA. Bem-estar psicológico, enfrentamento e lipodistrofia em pessoas vivendo com HIV/Aids. Psicol Estud. 2008;13(2):239-47.
11. Cuesta Gil M, Valverde Carrasco A, Duarte Ruiz B, Riba García F, Castrillo Tambay M, del Pino V. Utilización de polialcamida en cirugía reconstructiva y estética facial. Rev Esp Cir Oral y Maxilofac. 2007;29(6):367-74.
1. Plastic surgeon at the Plastic Surgery Department of the Universidade Federal de Juiz de Fora (HU-UFJF), full member of the Sociedade Brasileira de Cirurgia Plástica/ Brazilian Society of Plastic Surgery (SBCP), Juiz de Fora, MG, Brazil.
2. Plastic surgeon, full member of the SBCP, head of the Plastic Surgery Department of the University Hospital of the UFJF, Juiz de Fora, MG, Brazil.
3. Resident at the Plastic Surgery Department of the UFJF, Juiz de Fora, MG, Brazil.
4. Student of Medicine at the UFJF, Juiz de Fora, MG, Brazil.
5. Plastic Surgeon at the Plastic Surgery Department of the University Hospital of the UFJF, specialist member of the SBCP, Juiz de Fora, MG, Brazil.
6. Student of Medicine at the Faculty of Medical Sciences and Health of Juiz de Fora (Suprema), Juiz de Fora, MG, Brazil.
Correspondence to:
Marilho Tadeu Dornelas
Rua Dom Viçoso, 20 - Alto dos Passos
Juiz de Fora, MG, Brazil - CEP 36026-390
E-mail: marilho.dornelas@ufjf.edu.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: May 16, 2012
Article accepted: July 23, 2012
This study was performed at the Hospital Universitário da Universidade Federal de Juiz de Fora (HU-UFJF), Juiz de Fora, MG, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter