

Original Article - Year 2012 - Volume 27 -
Treatment of tuberous breast with combined incisions
Tratamento de mamas tuberosas com incisões combinadas
ABSTRACT
BACKGROUND: Tuberous breast deformity is a rare entity, first described by Rees and Aston in 1976. In this condition, breast development is altered, with herniation of the parenchyma through the nipple-areolar complex, enlargement of this structure, and hypoplasia of the breast tissue, especially in the lower quadrants. The breast thus acquires a tubular shape rather than the natural conical look.
METHODS: Four patients underwent a single surgical treatment, with combined inframammary and periareolar incisions. Technical details must be individualized for each case depending on the severity and classification type of the tuberous breasts.
RESULTS: The surgical procedure used covers all aspects of tuberous breast deformity in a single-stage operation. Revision of periareolar surgery scar was not necessary in any case. In all cases, the final aesthetic result was satisfactory for the patient and the surgeon. The procedure adopted does not interfere with future lactation.
CONCLUSIONS: Tuberous breast represents a real therapeutic challenge. The technique reported herein is very attractive and provides reliable and reproducible results.
Keywords: Breast/surgery. Mammaplasty. Plastic surgery/methods.
RESUMO
INTRODUÇÃO: A deformidade tuberosa da mama é uma rara entidade, descrita por Rees e Aston em 1976. O desenvolvimento mamário encontra-se alterado, com herniação do parênquima pelo complexo areolopapilar, alargamento dessa estrutura e hipoplasia do tecido mamário, principalmente nos quadrantes inferiores. A mama, portanto, adquire um aspecto tubular ao invés do aspecto cônico natural.
MÉTODO: No total, 4 pacientes foram submetidas a tratamento cirúrgico em um único tempo, com incisões combinadas: inframamária e periareolar. Detalhes técnicos devem ser individualizados para cada caso, conforme a gravidade e a classificação do tipo de mama tuberosa.
RESULTADOS: O procedimento cirúrgico utilizado aborda todos os aspectos da deformidade da mama tuberosa em operação de um estágio. Cirurgia de revisão de cicatriz periareolar não foi necessária em nenhum caso. Em todos os casos, obteve-se resultado estético final aceitável e com satisfação da paciente e do cirurgião. O procedimento adotado não interfere em lactações futuras.
CONCLUSÕES: A mama tuberosa representa um verdadeiro desafio terapêutico. A técnica utilizada é muito atraente e mostra resultados confiáveis e reprodutíveis.
Palavras-chave: Mama/cirurgia. Mamoplastia. Cirurgia plástica/métodos.
Tuberous breast deformity is a rare entity that affects young women either unilaterally or bilaterally. Its exact incidence and prevalence are unknown because of underdiagnosis and lack of clinical correlation in cases of breast asymmetry. It was first described by Rees and Aston1 in 1976. The deformity was so named for its similarity with the tuberous roots of plants.
Embryologically, breast development begins from the ectoderm in the fifth week of intrauterine life. Between 10 weeks and 14 weeks, the mammary buds of the thoracic region are surrounded by a layer of mesodermal tissue called the superficial fascia. Both glandular and nipple-areolar growth is complete at puberty, and the superficial fascia is the main structure responsible for forming conical breasts.
The formation of tuberous breasts stems from the lack of the upper section of the superficial fascia around the nipple-areolar complex and its thickening with the formation of a fibrous ring in the periareolar region. As a result, mammary development is altered, with pseudo-herniation of the parenchyma by the nipple-areolar complex and its enlargement as well as reduction of horizontal and vertical diameters with constriction of the base of the breast. Hypoplasia of the mammary tissue occurs, especially in the lower quadrants, with elevation of the inframammary fold (Figure 1). All these changes alter the conformation of the breasts, which acquire a tubular aspect, rather than the natural conical aspect1.
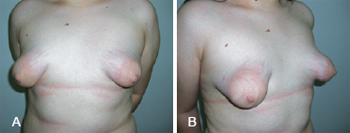
Figure 1 - A and B, tuberous breasts with hypoplasia of the mammary parenchyma and herniation through the extended nipple-areolar complex in frontal and profile views, respectively.
Moreover, asymmetry of the breasts is very common, with a prevalence of up to 89% in cases of tuberous breasts. Therefore, this condition should be carefully evaluated to optimize the surgical outcome, as it represents a major technical challenge. Some authors state that there is a deficiency of skin at the bottom of the breast, with the inframammary fold being larger than normal and in a higher position; this represents another technical difficulty in surgical treatment of tuberous breasts.
Tuberous breasts can be classified according to the severity of the malformation, which guides the choice of treatment. There are 2 main classifications: one by Grolleau et al.2 and the other by von Heimburg et al.3.
The classification of Grolleau et al.2 describes 3 types of tuberous breast deformities:
Type I - characterized by hypoplasia of the medial lower quadrant and the most common type, present in 54% of cases; Type II - both lower quadrants are hypoplastic, constituting 26% of cases; Type III - characterized by severe constriction, minimum based breast, and apparent deficiency of all quadrants of the breast, constituting the minority and present in 20% of cases.
The classification by von Heimburg et al.3 further divides the type II classification of Grolleau et al.2 into 2 types: type II, with normal subareolar skin; and type III, with deficient subareolar skin associated with parenchymal hypoplasia of the medial inferior and lateral quadrants. Therefore, the type I classification of von Heimburg et al.3 is equivalent to Grolleau et al.2 type I, and the type IV of von Heimburg et al.3 is equivalent to Grolleau et al.2 type III.
Subsequently, an objective definition of tuberous breast was advocated, using the Northwood index. This index is based solely on the herniation of breast parenchyma through the areola, which is a key point in the development of tuberous breast. The index is calculated by the relationship between the extent of parenchymal herniation through the areola (the distance from the tip of the nipple to the base of the areola, in centimeters) and areolar diameter (cm). An index greater than 0.4 defines a tuberous breast, which can be classified with respect to severity into light (0.4 to 0.5), moderate (0.51 to 0.6), or severe (0.61 to 0.7). The advantage of this classification in relation to those mentioned above is the objectivity it allows in defining and grading a tuberous breast4.
In this study, the authors present their experience with surgical repair of hypoplastic breasts; a single procedure was used to enlarge the breast and correct breast shape and size as well as the projection of the nipple-areolar complex.
History
Several surgical techniques have been suggested to correct deformities of tuberous breasts.
In 1976, the surgical treatment of tuberous breasts began with the technique described by Rees and Aston1; this technique employs radiating incisions in the breast parenchyma to release the fibrous ring and fix the shape and positioning of the breast through a port at the inframammary fold.
Ribeiro et al.5-7 described a technique that uses an exclusive periareolar approach with horizontal incisions in the parenchyma and preparation of the inferior pedicle base, with resection of the medial and lateral extensions and fixing of the same in the chest wall, folding the pedicle base on itself. This technique enables correction of the shape of the breasts by filling the lower hypoplastic quadrants.
In 2003, Mandrekas et al.8 advocated a similar approach, but with a vertical incision in the parenchyma in a 6 o'clock semi-axis position and its separation into 2 pillars. Since 1983, the use of combined periareolar and inframammary incisions associated with breast implants has been described by Teimourian and Adham9. Most patients with tuberous breast desire an increase in breast volume and improvement in shape, both of which are provided by this technique.
The authors of the current study present a procedure employed for treating tuberous breast with the use of combined incisions: an inframammary incision for placing a silicone breast implant and a periareolar incision for adjusting the size of the areola and achieving better treatment of the fibrous ring. This approach allows better control of hemostasis and less tissue trauma. It is also an easier technique, as it involves widespread access to the fibrotic pillars10.
METHODS
From January 2010 to January 2011, 4 patients with tuberous breasts were evaluated in the Plastic Surgery Service of the Universidade Federal do Triângulo Mineiro. All patients underwent surgical treatment with combined incisions at a single time.
Surgical markings were made preoperatively with the patient in a sitting or orthostatic position (Figure 2). The middle line was marked, and the desired size of the new nipple-areolar complex established, with an average diameter of 4 cm. The limits of dissection for implant accommodation were planned according to the projection of the breast parenchyma, and the ideal position of the new nipple-areolar complex was decided in relation to the sternal notch and its distance from the midline. The lateral boundary was coincident with the anterior axillary line. The medial boundary was generally positioned 2 to 3 cm lateral to the midsternal line. The upper limit was located approximately 4 to 5 cm below the clavicle. The lower limit was defined by the position of the new mammary sulcus, an average distance of 4 to 5 cm below the areola; a transverse incision 5-cm long was performed at this location. The positions of the inframammary ridges were determined to correspond to those on the normal contralateral side or to the position where the patient would wear her brassiere; if both sides were abnormal, the distance from the nipple or around 7 cm below the papilla was taken as a parameter.
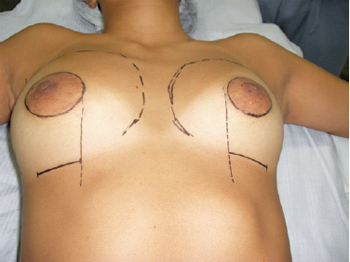
Figure 2 - Planning surgery: preoperative surgical demarcation, highlighting combined inframammary and periareolar incisions.
First, an inframammary incision was performed with detachment in the retroglandular subfascial plane for inclusion of silicone implants. Next, a periareolar incision was performed using an average areolatome of 4 cm in diameter. Incision at the outer limit of the areola, with de-epidermization between the areolar incisions, was carried out with a donut-type procedure (similar to the Schwartzman maneuver) to achieve adequate diameters of the areolas (Figure 3). Subsequently, an inferior semilunar incision was made with the concave side facing upwards in the breast parenchyma, with clear evidence of tissue herniation, followed by excision of the fibrous ring beams by 4 radiating incisions in the medial (position equivalent to 9 o'clock), lateral (position equivalent to 3 o'clock), top (position equivalent to 12 o'clock), and lower (position equivalent to 6 o'clock) quadrants (Figures 4 and 5). This technique enables communication of the periareolar access with the retroglandular space, allowing wide exposure of the surgical field and easy control of hemostasis.
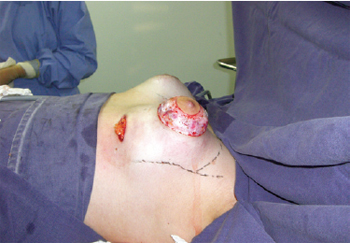
Figure 3 - Incision in the mammary fold with subfascial detachment, periareolar incision with delimitation of the neo-areola, and de-epidermization of areolar surplus. There is evident herniation of the fibrous ring.
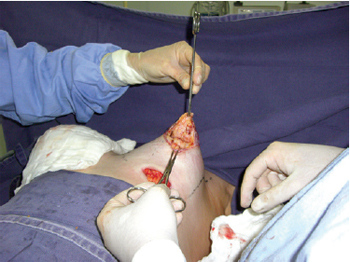
Figure 4 - Semilunar incision with the concave side facing upwards in the breast parenchyma, communication with the subfascial space, and incisions in 4 quadrants to break the fibrous ring.
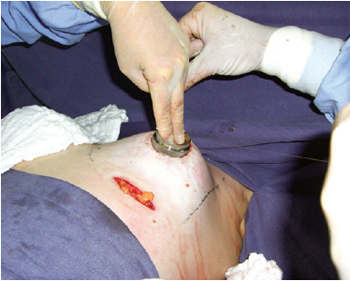
Figure 5 - Marking of the neo-areola with an average areolatome of 4 cm in diameter and distributed breast parenchyma.
Cohesive silicone gel implants with a textured surface, round base, and high or extra-high profile of various volumes that were specific to each patient (ranging from 200 to 265 mL) were introduced through the inframammary access route for achieving breast balance and symmetry (Figure 6).
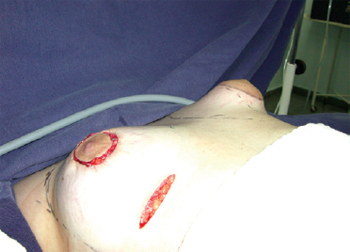
Figure 6 - Comparative evaluation of breast shape.
The inframammary incision was sutured in 3 planes: the subcutaneous plane, with 4-0 colorless nylon; the subdermal plane, with 4-0 monocryl and intradermal stitches of 3-0 nylon; and the periareolar plane, with round-block purse-string suture of 4-0 colorless nylon in 2 planes to adjust the skin on the areola, complemented with Benelli stitches of 5-0 nylon suture for mitigating the enlargement of the nippleareolar complex scar11,12 (Figure 7).
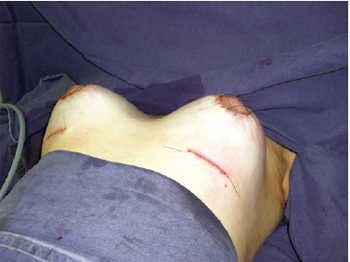
Figure 7 - Immediate postoperative appearance, showing combined incisions and placement of retroglandular breast implants.
At the end of the procedure, a compressive non-occlusive dressing was applied. An appropriate elastic compression bra was employed from the immediate postoperative period and maintained for 60 days. The first dressing change was performed after 48 hours. Physical exercise was allowed after 45 days.
Technical details must be individualized in each case for optimizing surgical outcome. In patients with normal-sized areolas, we chose a Webster type inferior hemiareolar incision, similar to that proposed for surgical correction of gynecomastia, rather than the periareolar incision. Moreover, in the presence of sagging skin associated with breast ptosis, an inverted T mastopexy incision was preferred (Figure 8), allowing resection of excess skin, better positioning of the nipple-areolar complex, and better shaping of the breasts.
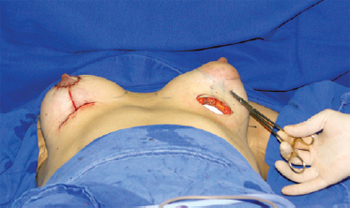
Figure 8 - Intraoperative image to highlight inverted T incision for resection of excess skin in cases of associated breast ptosis.
RESULTS
The first patient, who was 32 years of age, had asymmetric tuberous breasts (Grolleau type I on the left side and type II on the right). Implants with volumes of 215 mL and 235 mL were inserted in the left and right breasts, respectively (Figure 9).
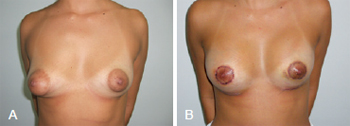
Figure 9 - In A, preoperative appearance of a patient with bilateral asymmetric tuberous breasts of Grolleau type I on the left and type II on the right. In B, postoperative appearance after addition of implants with volumes of 215 mL on the left and 235 mL on the right. Early postoperative period, 7 days after surgery.
The second patient presented a clinical peculiarity: she had Turner syndrome with bilateral Grolleau type III tuberous breasts and slight asymmetry. This patient was 23 years old and was treated with placement of bilateral 200 mL silicone implants (Figure 10).
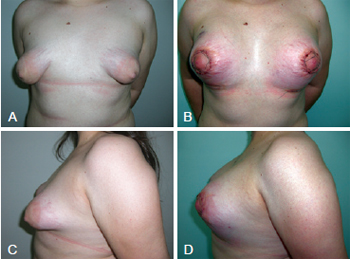
Figure 10 - In A and C, preoperative appearance of a patient with Turner syndrome, bilateral Grolleau type III tuberous breasts, and slight asymmetry. In B and D, early postoperative appearance 4 days after bilateral inclusion of 200 mL silicone implants.
In the third case, the patient was 25 years old and had symmetrical bilateral tuberous breasts of Grolleau type II with normal-sized areolas. In this patient, the Webster inferior hemiareolar incision was performed with placement of bilateral 235 mL implants (Figure 11).
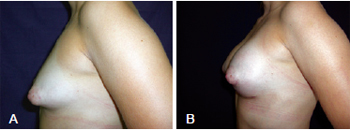
Figure 11 - In A, preoperative appearance of a patient with bilaterally symmetrical, Grolleau type II tuberous breasts and normal-sized areolas. In B, appearance in the late postoperative period 90 days after surgery. Inferior hemiareolar Webster incision was performed, with inclusion of 235 mL implants bilaterally.
The fourth patient, who was 39 years of age, had asymmetrical tuberous breasts with sagging skin and stretch marks as well as light ptosis of the left breast and moderate ptosis of the right breast. Correction of asymmetry required various skin resections because of the different degrees of ptosis between the 2 breasts. This allowed the inclusion of 2 equal 265 mL implants bilaterally (Figure 12).
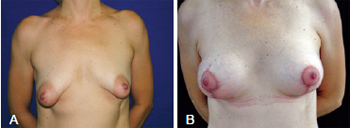
Figure 12 - In A, preoperative appearance of a patient with asymmetrical tuberous breasts with sagging skin and stretch marks as well as slight left and moderate right breast ptosis. In B, postoperative appearance at 14 days. The correction of asymmetry resulted from different skin resections for different degrees of ptosis between the 2 breasts, allowing the inclusion of equal 265 mL implants bilaterally.
In this study, 4 patients underwent a surgical procedure that addressed all aspects of their deformity in a single-stage operation. Surgical review for refinement or surgical scar revision was not necessary in any case.
In all cases, the final cosmetic result was satisfactory for both the patient and the surgical team. The degree of satisfaction was assessed subjectively.
There was one case of late enlargement of the nippleareolar complex without complaint from the patient; however, this was noticed by the surgeon.
The technique described has no functional consequences in the breasts, nor does it interfere with future lactation. No drains were used in any case. There were no complications such as seroma or hematoma.
Favorable results were based on the following aspects: normalization of the size of the areola, naturalization of breast contour and shape, good breast symmetry, and absence of residual tuberous deformity.
DISCUSSION
Tuberous breasts result from alteration of the superficial fascia, with restriction of radial mammary growth and tissue protrusion by the areolar segment, as well as enlargement of the areola.
Several surgical techniques for the treatment of tuberous breasts have been described in the past 30 years. Dinner & Dowden13 used a skin incision extending to the subcutaneous tissue and breast tissue in order to break the pillars, which were added to a flap of subcutaneous tissue. Other authors have tried to rearrange the lower breast pole with horizontal incisions in the parenchyma, with unsatisfactory cosmetic results. Ribeiro et al.5 described a technique in which the fibrous ring is incised horizontally with a rotation flap to fill the lower breast pole. Mandrekas et al.8 have proposed only one periareolar incision, in which vertical transection of the fibrous ring is achieved in the position equivalent to 6 o'clock, creating 2 lower pillars, with subsequent breast reassembly.
Pacifico & Kang4 described an alternative surgical approach, which combines reduction of the areola and placement of subglandular silicone implants. They state that tuberous breast deformity is exclusively due to areolar abnormality, and not due to the presence of a constrictive band.
Coleman & Saboeiro14 reported the benefits of autologous fat injection in the subcutaneous tissues of the breast and pectoral muscles. Good results were also achieved by Teimourian & Adham9 in 1983, using the technique of 2 incisions (areolar and submammary) with the use of prosthetics.
It is generally believed that the correction of tuberous breast deformity requires attention to several important points. The approach that is advocated by most authors is to outline a circumareolar pattern around the perimeter of the desired nipple-areolar complex15. A standard type of donut mastopexy is then performed. Following excision of the skin, the circumference of the surrounding tissues is incised. A silicone prosthesis is typically inserted into the pre-pectoral plane and covered entirely by parenchymal tissue. Some authors prefer submuscular implantation of the prosthesis because it reduces the risk of capsular contracture and provides better coverage of soft tissue prosthetics.
It is worth noting that the periareolar incision option is viable for the benefit of minimizing incisions, but entails the need for surgical review in up to 53% of cases in order to correct an enlarged periareolar scar16. Therefore, treatment with combined incisions is a procedure employed by the authors due to the advantages associated with inframammary incision, such as greater technical ease in preparing the retroglandular accommodation and inclusion of the breast implant, less tissue trauma, better exposure of the surgical field, and improved control of hemostasis. Additionally, the incision is inconspicuous, which facilitates establishment of the position of the new mammary ridge.
CONCLUSIONS
The tuberous breast represents a therapeutic challenge in physically and psychologically compromised patients. Symptoms at the onset of puberty hinder body image. A single surgical technique can be used to correct any degree of deformity of tuberous breasts17. Admittedly, the treatment of this deformity will continue to be a source of controversy, but we hope that this study will benefit patients with this condition. The reported procedure is performed in a single stage and can be used to treat all types of tuberous breasts, regardless of severity, with consistent surgical results. The technique is attractive and appears to be reliable and reproducible, achieving cosmetic results that are satisfactory for patients and surgeons.
REFERENCES
1. Rees TD, Aston SJ. The tuberous breast. Clin Plast Surg. 1976;3(2):339-47.
2. Grolleau JL, Lanfrey E, Lavigne B, Chavoin JP, Costagliola M. Breast base anomalies: treatment strategy for tuberous breasts, minor deformities, and asymmetry. Plast Reconstr Surg. 1999;104(7):2040-8.
3. von Heimburg D, Exner K, Kruft S, Lemperle G. The tuberous breast deformity: classification and treatment. Br J Plast Surg. 1996;49(6):339-45.
4. Pacifico MD, Kang VN. The tuberous breast revisited. J Plast Reconstr Aesthet Surg. 2007;60(5):455-64.
5. Ribeiro L, Canzi W, Buss A Jr, Accorsi A Jr. Tuberous breast: a new approach. Plast Reconstr Surg. 1998;101(1):42-50.
6. Ribeiro L, Accorsi A Jr, Buss A, Pessĵa MC. Short scar correction of the tuberous breast. Clin Plast Surg. 2002;29(3):423-31.
7. Ribeiro L, Accorsi A Jr, Buss A, Marcal-Pessoa M. Creation and evolution of 30 years of the inferior pedicle in reduction mammaplasties. Plast Reconstr Surg. 2002;110(3):960-70.
8. Mandrekas AD, Zambacos GJ, Anastasopoulos A, Hapsas D, Lambrinaki N, Ioannidou-Mouzaka L. Aesthetic reconstruction of the tuberous breast deformity. Plast Reconstr Surg. 2003;112(4):1099-108.
9. Teimourian B, Adham MN. Surgical correction of the tuberous breast. Ann Plast Surg. 1983;10(3):190-3.
10. Sabino Neto M, Silva AL, Garcia EB, Freire M, Ferreira L. Quality of life and self-esteem after breast asymmetry surgery. Aesthet Surg J. 2007;27(6):616-21.
11. Peled IJ, Zagher U, Wexler MR. Purse-string suture to reduction and closure of skin defects. Ann Plast Surg. 1985;14(5):465-9.
12. Benelli L. Technique personnelle de plastie mammaire péri-aréolaire: le 'round-block'. Cah Chir. 1991;77(1):15-25.
13. Dinner MI, Dowden RV. The tubular / tuberous breast syndrome. Ann Plast Surg. 1987;19(5):414-20.
14. Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119(3):775-87.
15. DeLuca-Pytell DM, Piazza RC, Holding JC, Snyder N, Hunsicker LM, Phillips LG. The incidence of tuberous breast deformity in asymmetric and symmetric mammaplasty patients. Plast Reconstr Surg. 2005;116(7):1894-9.
16. Argenta LC, VanderKolk C, Friedman RJ, Marks M. Refinements in reconstruction of congenital breast deformities. Plast Reconstr Surg. 1985;76(1):73-82.
17. Reynaud JP, Gary-Bobo A, Baron JL, Bousquet P, Dessus B. Tuberous breast: clinical and therapeutic considerations. Report of 20 cases. Ann Chir Plast Esthet. 1990;35(6):453-8.
1. Full member of the Sociedade Brasileira de Cirurgia Plástica/ Brazilian Society of Plastic Surgery (SBCP), Supervisor of the Plastic Surgery Service, Universidade Federal do Triângulo Mineiro (UFTM), Uberaba, MG, Brazil.
2. Resident Physician of the Plastic Surgery Service, UFTM, Associated Member of the SBCP, Uberaba, MG, Brazil.
3. Resident Physician of the Plastic Surgery Service, UFTM, Uberaba, MG, Brazil.
4. Member of the SBCP, Uberaba, MG, Brazil.
Correspondence to:
Manoel Pereira Silva Neto
Rua Dona Rafa Cecílio, 445 - Vila Maria Helena
Uberaba, MG, Brazil - CEP 38020-080
E-mail: drmanoel@drmanoel.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: June 15, 2012
Article accepted: August 7, 2012
This study was performed at the Universidade Federal do Triângulo Mineiro, Uberaba, MG, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter