

Original Article - Year 2013 - Volume 28 -
Use of the Pressure Ulcer Scale for Healing tool to evaluate the healing of chronic leg ulcers
Uso da ferramenta Pressure Ulcer Scale for Healing para avaliar a cicatrização de úlcera crónica de perna
ABSTRACT
INTRODUCTION: In the present study, we aimed to describe the evolution of the healing of chronic leg ulcers using the Pressure Ulcer Scale for Healing (PUSH) tool.
METHODS: The data were collected from July 2010 to May 2011. The inclusion of patients in the study followed the order of arrival. The lesion was evaluated weekly according to the PUSH tool.
RESULTS: The study included 15 (30%) patients with diabetes and foot ulcers and 35 (70%) patients with venous ulcers. At the beginning of the data collection process, the average ulcer length and width were 9.26 cm2 (range, 12.1-24.0 cm2). At 9 months of treatment, the average ulcer length and width was 2.04 cm2 (range, 0.3-0.6 cm2). At the beginning of the study, the average amount of exudate was 1.71 (moderate amount), whereas at 9 months after the beginning of treatment, the average amount of exudate was 0.14 (absence of exudate). At 9 months of treatment, 19 (38%) patients had closed ulcers, 17 (34%) had ulcers with granulation tissue, and 14 (28%) had ulcers with epithelialized tissue.
CONCLUSIONS: Use of the PUSH tool enabled monitoring of the ulcer healing process through the evaluation of length vs. width, exudate amount, and type of tissue present in the wound, thus favoring the selection of the correct dressing for each stage of wound healing.
Keywords: Leg ulcer. Diabetic foot. Varicose ulcer. Wound healing. Nursing assessment.
RESUMO
INTRODUÇÃO: O objetivo deste estudo é descrever a evolução da cicatrização de úlcera crônica de perna, utilizando o instrumento Pressure Ulcer Scale for Healing (PUSH).
MÉTODO: Os dados foram coletados no período de julho de 2010 a maio de 2011. A inclusão dos pacientes no estudo obedeceu à ordem de chegada. A lesão foi avaliada semanalmente, sendo aplicada a escala PUSH.
RESULTADOS: Foram incluídos no estudo 15 (30%) pacientes diabéticos com pé ulcerado e 35 (70%) pacientes com úlcera venosa. No início da coleta dos dados, a média do comprimento e da largura foi de 9,26, caracterizando que a lesão mensurava de 12,1 cm2 a 24 cm2. Com 9 meses de tratamento, a úlcera apresentou média de comprimento e de largura de 2,04, caracterizando que a lesão mensurava de 0,3 cm2 a 0,6 cm2. Com relação à quantidade do exsudato, no início da coleta de dados a média foi de 1,71, caracterizando que a lesão apresentava quantidade moderada e, 9 meses após o início do tratamento, houve redução do exsudato, com média de 0,14, significando ausência de exsudato. Aos 9 meses de tratamento, 19 (38%) pacientes apresentavam úlcera fechada; 17 (34%), úlceras com tecido de granulação; e 14 (28%), tecido epitelizado.
CONCLUSÕES: O instrumento PUSH possibilitou acompanhar o processo de cicatrização da lesão por meio da avaliação de comprimento versus largura, quantidade do exsudato e tipo de tecido existente na ferida, favorecendo, assim, a escolha da cobertura ideal para cada fase da cicatrização.
Palavras-chave: Úlcera da perna. Pé diabético. Úlcera varicosa. Cicatrização. Avaliação em enfermagem.
Although patients with wounds are part of a special group due to their common characteristics, they are individuals with needs and whose reactions are dependent on their own identity and subjectivity. Thus, the answer to the problems caused by skin disruption is related to their specific condition, such as family and financial support and assistance received in all phases of treatment, since these individuals often experience pain, presence of exudate and odor, prejudice, and isolation from family and friends1.
The incidence and prevalence of chronic ulcers are both very high and result in high financial costs for both patients and society as well as social, emotional, and psychological consequences for the patient. Therefore, the development of new approaches in this area is required to enhance the features and technologies of wound treatment to make it more affordable and accessible, especially to the less privileged economic classes and patients in less developed societies with fewer financial resources2.
In Brazil, wounds affect individuals throughout the population regardless of gender, age, or ethnicity and create alterations in skin integrity in larger numbers of individuals; thus, it constitutes a serious public health problem. However, there are no statistical data to corroborate this fact due to the scarcity of records of these cases. However, these wounds affect government spending and also affect the quality of life throughout the Brazilian population3.
Although it is a systemic process, tissue healing requires topical therapy that is suitable for promoting the physiological process4. Therefore, it is necessary for medical professionals to know how to evaluate wounds and apply optimal dressing according tissue and exudate type1-3.
Skin care knowledge is essential for improving the quality of life of people through interventions that accelerate healing time, reduce risks, complications, and pain, and optimize the cost/benefit for the treatment of acute injuries, especially if they are chronic in more susceptible patients, such as elderly individuals and those with diabetes5. Therefore, it is important that health professionals use an assessment tool that will give parameters that assist with the selection of the right dressing for each phase of the healing process.
The process of wound evaluation is of fundamental importance for the development of a good therapeutic plan. The effectiveness of local treatment and injury assessment can occur only once the interventional observations and results are documented6. The evaluation of a wound can cause varied interpretations due to the diversity of its nature, form, and location together with the perception by each nurse due to knowledge differences among professionals. The same wound can be evaluated and have different registries that can generate conflicting or differing interpretations7. Among the evaluated parameters are the anatomical location; lesion size; color; damaged tissue type and extension; presence of foreign matter, fistulae, and tunnels; skin condition around the wound; and exudate characteristics3,8,9. This assessment helps the healthcare provider perform the appropriate action to aid with wound healing. It is important that the professional uses a tool that enables monitoring and evaluation of injury during the healing process.
In the present study, we aimed to describe the evolution of the healing of chronic leg ulcers using the Pressure Ulcer Scale for Healing (PUSH) tool.
METHODS
This clinical, descriptive, and analytical study was performed at the Plastic Surgery Clinic, São Paulo Hospital - Wounds Ward and Wound Clinic of Sorocaba Hospital. The data were collected from July 2010 to May 2011 after approval was granted by the Ethics Committee of São Paulo Federal University (CEP 0793/10). Patients were enrolled in the study in the order of arrival. Each lesion was evaluated weekly for 9 months according to PUSH tool criteria10. A total of 50 patients with chronic leg wounds were included in the study.
The inclusion criteria were age > 18 years and the presence of chronic wounds in the lower limbs. The exclusion criteria were: oral or visual impairment or the presence of ulcers in anatomical locations other than the lower limbs.
The PUSH tool, which was used to assess the wounds, uses 3 parameters to evaluate the wound healing process and intervention outcomes. The first parameter is the area of the wound, which is measured in terms of longest length (in the cephalocaudal direction) versus largest width (in the horizontal line from right to left) in square centimeters. The wound area is obtained by the multiplication of values of 0-24 cm2 and scores of 0-10, according to the area obtained. The second parameter refers to the amount of exudate present on the wound after removal of the dressing and prior to the application of any topical agent. Exudate amount is classified as absent, small, moderate, or large, which correspond to scores of 0 (absent) to 3 (large), respectively. The third parameter is the appearance of the wound bed, which is defined as the type of tissue present in this region and is specified as: necrotic tissue (eschar), black, brown, or chestnut coloration that adheres firmly to the wound bed or edges and may appear hardened or softened compared to the peripheral skin; sloughing yellow or white tissue that adheres to the wound bed and is present as strings or thick crusts that may be mucinous; granulation tissue that is pink or reddish in color with a bright, moist, and grainy appearance; epithelial tissue that appears as new bright or pink tissue that develops from the edges or as "islands" on the lesion surface (superficial wounds); and a closed or covered wound that is completely covered with epithelium. These fabrics correspond to the scores 0 (closed wound), 1 (epithelial tissue), 2 (granulation tissue), 3 (slough), and 4 (necrotic tissue).
The summed sub-scores for these parameters or subscales yield a total score of 0-17. Higher scores indicate worse ulcer condition, while lower scores indicate improved healing. Therefore, measuring only 3 variables, the PUSH tool generates PUSH scores, which can describe the ulcer condition and healing progress. This tool was created to monitor the progress of pressure ulcers, but it has been adapted and validated in Brazil for monitoring leg ulcers5,6,10.
The statistical analysis was performed using the chi-square, Friedman, and Dunn tests with a significance level of < 5% (P < 0.05).
RESULTS
This study a total of 50 patients, including 15 (30%) patients with diabetes and foot ulcers and 35 (70%) patients with venous ulcers. Thirty (60%) patients were smokers, but no statistical differences were detected between smokers and nonsmokers (P = 0.157).
Table 1 shows that 21 (42%) of the respondents were 65-73 years of age and 19 (38%) were 60-64 years of age. The mean age was 63.70 years. With regard to gender, 35 (70%) were females; the difference between the number of males and females was statistically significant (P = 0.0007). With regard to occupation, 26 (52%) patients were retired and 13 (26%) were unemployed, the difference between which was statistically significant (P = 0.0019).
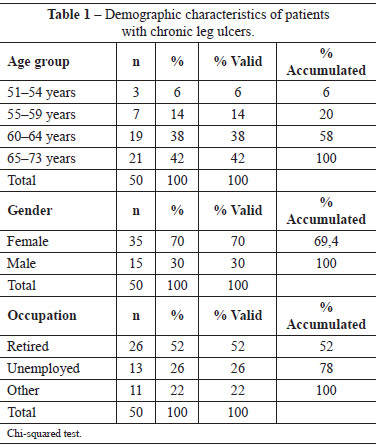
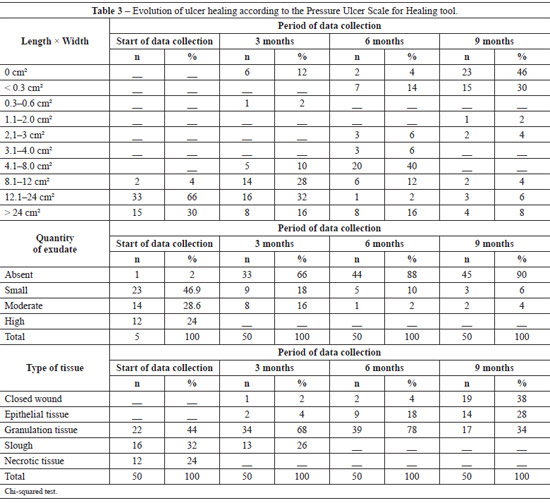
Table 2 shows the ulcer evolution using the PUSH tool. At the beginning of the data collection process, the average wound length and width was 9.26 cm2 (range, 12.1-24.0 cm2); the average wound lengths after 3 months, 6 months, and after 9 months of treatment were 7.46 cm2 (range, 4.1-8.0 cm2), 6.34 cm2 (range, 3.1-4.0 cm2), and 2.04 cm2 (range, 0.3-0.6 cm2) (P < 0.001), respectively.
At the beginning of the data collection process, the average exudate score was 1.71, characterizing a lesion with moderate amount of exudate; 9 months after treatment initiation, the average exudate score had reduced to 0.14, which characterized a lesion with the absence of exudate (P < 0.001). At the baseline, the average tissue type was 3, which corresponds to sloughing tissue. After 9 months of treatment, the average ulcer score was 0.96, which corresponded to the presence of epithelial tissue.
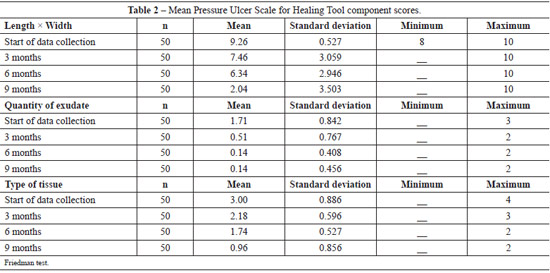
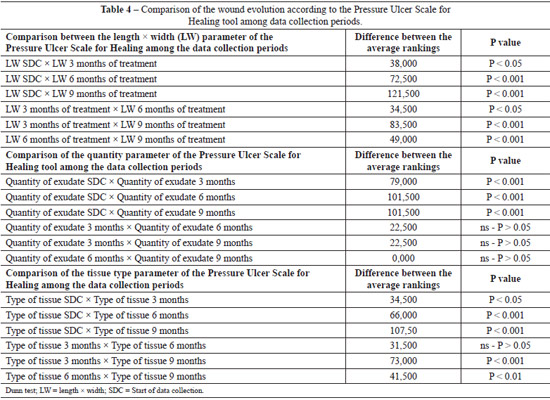
Table 3 shows the evolution of the wound healing process. At the beginning of the data collection process, 33 (66%) of the ulcers were 12.1-24 cm2 in size and 15 (30%) were > 24 cm2 in size. Slight improvement was noted after 3 months of treatment: 16 (32%) ulcers were 12.1-24 cm2 in size and 14 (28%) were 8.1-12 cm2 in size. At 6 months of treatment, significant improvement was noted: 20 (40%) ulcers were 4.1-8 cm2 in size, and after 9 months of treatment, 23 (46%) ulcers were 0 cm2 in size and 15 (30%) ulcers were < 0.3 cm2 in size.
At baseline, 23 (46.9%) ulcers had a small amount of exudate and 14 (28.6%) ulcers had a moderate amount of exudate. After 3, 6, and 9 months of treatment, 33 (66%), 44 (88%), and 45 (90%) lesions had no exudate, respectively.
At the beginning of the data collection process, 16 (32%) ulcers had sloughing tissue and 12 (24%) ulcers had necrotic tissue. With 3 months of treatment, 34 (68%) lesions had granulation tissue and 13 (26%) lesions had sloughing tissue. At 6 months of treatment, a significant improvement was noted, with 39 (78%) ulcers showing granulation tissue and 9 (18%) showing epithelialized tissue. After 9 months of treatment, 19 (38%) patients had closed ulcers, 17 (34%) had ulcers with granulation tissue, and 14 (28%) had ulcers with epithelialized tissue and were < 0.3 cm2 in size.
Table 4 compares the parameters that comprise the PUSH tool according to the data collection periods (beginning and 3, 6, and 9 months of treatment). Statistically significant differences in parameter length compared with width were noted among the data collection periods. With regard to exu-date amount, the initial data collection period values were statistically significant from those of all other data collection periods (3, 6, and 9 months of treatment); however, there were no significant differences among the other periods. As for tissue type, we observed that only the comparisons between the periods of data collection (beginning of data collection vs. 3, 6, and 9 months of treatment) indicated a statistically significant difference (P > 0.05). All other comparisons indicated statistically significant differences.
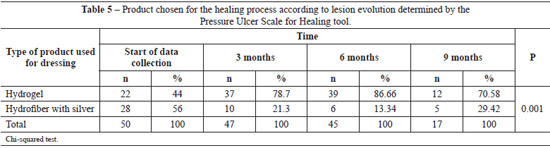
Using the PUSH tool to evaluate the wound healing process, we observed reductions in lesion length and width, decreased amounts of exudate, and improved tissue quality throughout the wound healing process. The use of this tool enables the identification of the ideal dressing product for each phase of the healing process. At the beginning of the data collection period, 22 (44%) lesions were treated with hydrogel and 28 (56%) were treated with hydrofiber with silver. At 3, 6, and 9 months of treatment, hydrogel was used in the majority of the ulcers (Table 5).
DISCUSSION
Lower limb ulcers are common in patients with chronic diseases, especially those related to the circulatory system and diabetes mellitus11,12. In Brazil, wounds are a serious public health problem due to the large number of people with chronic and degenerative diseases; however, there is no record of the number of individuals with wounds. It is estimated that 15% of patients with diabetes mellitus will develop at least one foot injury throughout their lifetime and that among patients suffering from chronic venous insufficiency, 0.5-1.5% will develop a venous ulcer13-16.
This study involved 15 (30%) patients with diabetes and foot ulcers and 35 (70%) patients with venous ulcers, the majority of whom were women > 60 years of age. These findings corroborate the results of several national and international studies3,5,12,15,16.
Increasing age is a systemic factor that can negatively impact the healing process. Physiologically, with age, there is a reduction in the metabolic processes of cell proliferation, collagen production, and healing velocity. Thus, it is expected that individuals in older age groups heal more slowly than those in younger age groups17. In the present study, we noted that 26 (52%) study participants were retired and 13 (26%) were unemployed. Venous ulcers often cause patients to leave or retire from work since they often remain open for months or years, which has a socioeconomic impact in terms of treatment cost and quality of life18,19.
Thirty (60%) participants in this study were smokers. Smoking damages tissue oxygenation, reduces the body's resistance and leaves it more susceptible to infection, and slows the healing process. Smoking also alters collagen synthesis, thus hindering wound healing20,21. Moreover, smoking reduces hemoglobin function and causes lung dysfunction, thereby predisposing an individual to oxygen deprivation. Nicotine causes vasoconstriction, which increases the risk of ischemia and the development of ulcers; in fact, healing of preexisting ulcers is reported to be difficult22,23. In such cases, the cellular process is disrupted and abnormal functions occur due to systemic, local, or both factors in the healing process.
The wound treatment process begins with their assessment and documentation, and healthcare professionals must always remember that each patient and every wound is unique. These assessments should be made prior to the planning and implementation of therapeutic interventions24. The completion of a treatment plan, as well as the skill of the professional who treats the wound, determines the effectiveness of the chosen product that is intended to promote an ideal environment to stimulate ulcer healing. The success of a patient's treatment plan depends on the individual's complete history as well as regular assessments of systemic factors and wound sites 25,26.
Nurses working with patients who have wounds should evaluate them to judge their evolution, and this assessment should contain objective measurements that are reviewed periodically after the initial assessment. The evaluation of a lesion should include the following: exudate, tissue type, lesion size, lesion margin and center, the presence of pain and odors, and any signs of infection. After these parameters are evaluated, the professional must choose the ideal dressing for promoting the healing process. All observations should be recorded systematically to ensure high-quality and humane nursing care.
In the care of patients with wounds, the evaluation of the evolution of the lesion should be performed using determined criteria based on tools that facilitate annotation of the wound characteristics as well as factors that can slow this process27-29. This tool should enable professionals to monitor the lesions and, therefore, assess the effect of an intervention. Assessment tools that enhance and stimulate communication among professionals enable them to achieve the expected goals24-26,28,29.
The use of the PUSH tool in the current study made it possible to monitor the wound healing process since it involved recording the reduction in lesion size. At the beginning of the data collection process, 33 (66%) ulcers were 12.1-24 cm2 in size. After 9 months of treatment, 15 (30%) ulcers were > 24 cm2, 23 (46%) were 0 cm2, and 15 (30%) were < 0.3 cm2 in size (which was considered lesion closure).
Ratliff & Rodeheaver30 conducted a study using the PUSH tool that included 27 patients with venous ulcers. The study lasted 2 months and included 23 patients. In the first evaluation, the total PUSH score was 12 points. At the 1 month follow-up (end of the study), the total PUSH score was 8 points, indicating that the ulcers had healed.
In another study using the PUSH tool that described the evolution of the healing process in 2 patients with diabetes and foot ulcers, the authors concluded that this instrument eases the nursing burden since is based on the evaluation of important parameters involved in careful dynamic wound care. It also facilitated the observation of the evolution of the healing process, thus allowing the professional to choose of the optimal dressing for each phase of the wound healing process31.
Regarding the amount of exudate at the beginning of the data collection process, 23 (46.9%) ulcers had small amounts of exudate and 14 (28.6%) had moderate amounts of exudate. After 9 months of treatment, 45 (90%) ulcers had any exudate. With respect to the type of tissue, in the beginning of data collection, 16 (32%) ulcers had sloughing tissue and 12 (24%) patients had necrotic tissue. With 9 months of treatment, 19 (38%) patients had closed ulcers, 17 (34%) patients had ulcers with granulated tissue, and 14 (28%) had ulcers with epithelial tissue.
In a study that included 18 patients with diabetes and foot ulcers who were followed for 13 weeks, the authors concluded that, using the PUSH tool, it is possible to monitor the healing process and choose a suitable dressing product32. The results of another study including 98 patients with venous and diabetic ulcers suggest that PUSH is a simple tool and that it comprises all the items needed for monitoring and documenting the wound healing process9.
Several studies have found that the PUSH tool guides the professional's clinical reasoning beyond stage identification and healing process evolution to encourage high-quality and effective care, and allows the professional to choose the most appropriate dressing for the wound healing process30-34.
To ensure that the healing process occurs in a proper and orderly manner, the professional thoroughly evaluates the wound and identifies all inflammatory agents that must be removed from the wound bed by thorough cleaning. After this procedure, the professional must choose the optimal dressing to keep the wound moist35,36.
Healing is optimized and the potential for infection is minimized when all necrotic tissue, exudate, and metabolic debris are removed from the wound. The cleaning process involves careful selection of both the solution and the method with ample consideration of the benefits to the patient and for minimizing wound-related trauma37,38.
The healing process requires topical treatment of the lesion through wound dressing and cleaning. It has been proven that as an injury is covered, it forms a physical barrier between the injured wound bed and the external environment that provides some of the ideal principles for rapid healing such as humidity and temperature. The choice of dressing for wound treatment should consider its ability to prevent infection37,38.
In the present study, we observed reduced lesion length and width, decreased amounts of exudate, and tissue improvement. Therefore, we were able to select the ideal product to keep the wound moist and stimulate the healing process. At the beginning of the data collection process, 22 (44%) devitalized wounds were treated with hydrogel, while 28 (56%) wounds were treated with hydrofiber with silver. Over 3, 6, and 9 months of data collection, the hydrogel dressing was used in most of the ulcers.
Wound dressings are a form of treatment, and their selection depends on intrinsic and extrinsic factors. The treatment of wounds is dynamic and depends on the healing stages39. There are currently numerous choices of dressings that are commercially available. The financial resources of the patient and/or health facility; the need for continued use of the dressing including home visits; an evaluation of the benefits and costs; and the wound nature, size, and location are some of the aspects to be considered during the dressing selection process. Although a great variety of dressings are available, only one type of dressing does not meet the requirements to be applied in all types of skin wounds39.
Healing under moist conditions has the following advantages compared to dry environments: preventing dehydration of the tissue, which leads to cell death; accelerating angiogenesis; stimulating epithelialization and granulation tissue formation; facilitating the removal of necrotic tissue and fibrin; serving as a protective barrier against microorganisms; promoting the reduction of pain; and preventing excessive fluid loss and trauma during dressing changes31,40.
Hydrofiber is an anti-microbial dressing with silver that contains sodium carboxymethylcellulose and 1.2% ionic silver. It is absorbent and able to capture any microorganisms that are present in the wound bed. Upon contact with exudate, the dressing becomes a cohesive gel. Hydrofiber with silver maintains a humid environment and controls bacteria, thus contributing to the body's healing process and reducing the risk of infection31.
Work performed by several authors on patients with chronic and acute wounds showed that hydrofiber with silver works by slowing the exudate, by acting as a chemical debridement that liquefies all devitalized and necrotic tissue, and has a bactericidal effect that stimulates granulated tissue development and promotes healing41,42.
The use of hydrogel is indicated for dry wounds or those with minimal exudate, granulation tissue, and necrosis since it aids in the removal of crusts. It can also be used in clean superficial laceration wounds such as cuts, abrasions, donor and acceptor graft sites, diabetic ulcers, pressure ulcers, ulcers in the lower limbs (arterial, venous, and mixed), and first and second degree burns. Hydrogel also has chemotactic action for leukocytes, promotes angiogenesis, promotes autolytic debridement, and maintains ideal humidity for the healing process42.
CONCLUSIONS
The use of the PUSH tool in the current study allowed for the monitoring of the wound healing process through evaluations of the length vs. width, exudate amount, and tissue type within the wound, thus allowing the selection of the ideal dressing for each stage of healing.
REFERENCES
1. Morais GFC, Oliveira SHS, Soares MJGO. Avaliação de feridas pelos enfermeiros de instituições hospitalares da rede pública. Texto & Contexto Enferm. 2008;17(1):98-105.
2. Mandelbaum SH, Di Santis EP, Mandelbaum MHS. Cicatrização: conceitos atuais e recursos auxiliares. Parte I. An Bras Dermatol. 2003;78(4):393-410.
3. Salomé GM, Maria de Souza Pellegrino D, Blanes L, Ferreira LM. Self-esteem in patients with diabetes mellitus and foot ulcers. J Tissue Viability. 2011;20(3):100-6.
4. Matsui Y, Furue M, Sanada H, Tachibana T, Nakayama T, Sugama J, et al. Development of the DESIGN-R with an observational study: an absolute evaluation tool for monitoring pressure ulcer wound healing. Wound Repair Regen. 2011;19(3):309-15.
5. Santos VLCG, Carvalho VF. Reapresentando o instrumento Pressure Ulcer Scale for Healing (PUSH) para avaliação de úlcera por pressão e úlcera crônica de perna. Rev Estima. 2009;7(2):19-27.
6. Santos VLCG, Sellmer D, Massulo MME. Confiabilidade interobservadores do Pressure Ulcer Scale for Healing (PUSH), em pacientes com úlceras crônicas de perna. Rev Latino-Am Enfermagem. 2007;15(3):391-6.
7. Sung YH, Park KH. Factors affecting the healing of pressure ulcers in a Korean acute care hospital. J Wound Ostomy Continence Nurs. 2011;38(1):38-45.
8. George-Saintilus E, Tommasulo B, Cal CE, Hussain R, Mathew N, Dlugacz Y, et al. Pressure ulcer PUSH score and traditional nursing assessment in nursing home residents: do they correlate? J Am Med Dir Assoc. 2009;10(2):141-4.
9. Hon J, Lagden K, McLaren AM, O'Sullivan D, Orr L, Houghton PE, et al. A prospective, multicenter study to validate use of the PUSH in patients with diabetic, venous, and pressure ulcers. Ostomy Wound Manage. 2010;56(2):26-36.
10. Santos VLCG, Azevedo MAJ, Silva TS, Carvalho VMJ, Carvalho VF. Adaptação transcultural do pressure ulcer scale for healing (PUSH) para a língua portuguesa. Rev Latino-Am Enfermagem. 2005;13(3):305-13.
11. Lucas LS, Martins JT, Robazzi MLCC. Qualidade de vida dos portadores de ferida em membros inferiores: úlcera de perna. Ciênc Enferm. 2008;14(1):43-52.
12. Salomé GM, Blanes L, Ferreira LM. Avaliação de sintomas depressivos em pessoas com diabetes mellitus e pé ulcerado. Rev Col Bras Cir. 2011;38(5):327-33.
13. Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes management cohort. Diabetes Care. 2003;26(5):1435-8.
14. Torquato MT, Montenegro Júnior RM, Viana LA, Souza RA, Lanna CM, Lucas JC, et al. Prevalence of diabetes mellitus and impaired glucose tolerance in the urban population aged 30-69 years in Ribeirão Preto (São Paulo), Brasil. São Paulo Med J. 2003;121(6):224-30.
15. Abbade LPF, Lastória S. Abordagem de pacientes com úlcera de perna de etiologia venosa. An Bras Dermatol. 2006;81(6):509-22.
16. Clarke-Moloney M, O'Brien JF, Grace PA, Burke PE. Health-related quality of life during four-layer compression bandaging for venous ulcer disease: a randomised controlled trial. Ir J Med Sci. 2005;174(2):21-5.
17. Côrtes SMS, Alvarez RRA. Avaliação da cicatrização estimulada por aceleradores em pacientes adultos com hanseníase, portadores de úlcera plantares. Rev Nursing. 2011;14(159):434-9.
18. Franco D, Gonçalves LF. Feridas cutâneas: a escolha do curativo adequado. Rev Col Bras Cir. 2008;35(3):203-6.
19. White R, Mcintosh C. Topical therapies for diabetic foot ulcers: standard treatments. J Wound Care. 2008;17(10):426-32.
20. Thomsen T, Tonnesen H, Moller AM. Effect of preoperative smoking cessation interventions on postoperative complications and smoking cessation. Br J Surg. 2009;96(5):451-61.
21. Kean J. The effects of smoking on the wound healing process. J Wound Care. 2010;19(1):5-8.
22. Lindström D, Wladis A, PekkariK. The thioredoxin and glutaredoxin systems in smoking cessation and the possible relation to postoperative wound complications. Wounds. 2010;4(1):88-93.
23. Gottrup F, Apelqvist J, Price P. Outcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care. 2010;19(6):237-68.
24. Aron S, Gamba MA. Preparo do leito da ferida e a história do TIME. Rev Estima. 2009;7(4):20-4.
25. Salomé GM. Hidrofibra com prata e hidrogel com alginato na cicatrização de ferida em paciente com diabetes mellitus. Rev Estima. 2008;6(4):28-32.
26. White R, Mcintosh C. A review of the literature on topical therapies for diabetic foot ulcers. Part 2: advanced treatments. J Wound Care. 2009;18(8):335-41.
27. Bajay HM, Pedrosa MMO, Lourenço MTN, Cortez SL, Paula MAB. Registro de avaliação e evolução de feridas: subsídios para reflexão e mudanças. Rev Estima. 2003;1(2):20-9.
28. Salomé GM, Blanes L, Ferreira LM. Capacidade funcional dos pacientes com diabetes mellitus e pé ulcerado. Acta Paul Enferm. 2009;22(4):412-6.
29. Jones JE, Robinson J, Barr W, Carlisle C. Impact of exudate and odour from chronic venous leg ulceration. Nurs Stand. 2008;22(45):53-4.
30. Ratliff CR, Rodeheaver GT. Use of the PUSH tool to measure venous ulcer healing. Ostomy Wound Manage. 2005;51(5):58-60.
31. Salomé GM, Araújo VS. Uso do Pressure Ulcer Scale for Healing (PUSH) no acompanhamento da cicatrização em paciente diabético com úlcera no pé. Rev Nursing. 2010;14(149):507-11.
32. Gardner SE, Hillis SL, Frantz RA. A prospective study of the PUSH tool in diabetic foot ulcers. J Wound Ostomy Continence Nurs. 2011;38(4):385-93.
33. Bonham PA, Kelechi T, Mueller M, Robinson J. Are toe pressures measured by a portable photophlethysmograph equivalent to standard laboratory tests? J Wound Ostomy Continence Nurs. 2010;37(5):475-86.
34. Sung YH, Park KH. Factors affecting the healing of pressure ulcers in a Korean acute care hospital. J Wound Ostomy Continence Nurs. 2011;38(1):38-45.
35. Ferreira AM, Neves DDS, Silva APM, Felício NB. Limpeza de feridas que cicatrizam por segunda intenção: a prática dos profissionais de enfermagem. Rev Estima. 2003;1(3):25-30.
36. Leaper D. Leg ulcers. Antiseptics and their effect on healing tissue. Nurs Times. 1986;82(22):45-7.
37. Leaper DJ. Silver dressings: their role in wound management. int Wound J. 2006;3(4):282-94.
38. Salomé GM. Avaliando lesão: prática e conhecimentos dos enfermeiros que prestam assistência ao indivíduo com ferida. Saúde coletiva. 2009;35(6):280-7.
39. Broughton G 2nd, Janis JE, Attinger CE. A brief history of wound care. Plast Reconstr Surg. 2006;117(7 Suppl):6S-11S.
40. Morgan DA. Wound dressings: principals and types of dressings. in: Formulary of wound management products: a guide for health care staff. 6th ed. Haslemere: Euromed Communications; 1994. p.64-73.
41. Salomé GM,Arbage CC.Aplicabilidade da hidrofibra com prata em lesão provocada pela síndrome da Fournier: relato de experiência. Nursing. 2008;11(127):566-70.
42. Mandelbaum SH, Di Santis EP, Mandelbaum MHS. Cicatrização: conceitos atuais e recursos auxiliares. Parte II. An Bras Dermatol. 2003;78(5):521-2.
1. Medical student of the University of Vale do Sapucaí (UNIVAS), Pouso Alegre, MG, Brazil
2. Nurse, Auditing Expert, São Paulo, SP, Brazil
3. Postdoc, Professor of the Professional Master's Degree in Applied Health Sciences of the UNIVAS, Pouso Alegre, MG, Brazil
4. Full professor of the Plastic Surgery Discipline of the Escola Paulista de Medicina-Universidade Federal de São Paulo (EPM-UNIFESP), full member of the Sociedade Brasileira de Cirurgia Plástica/Brazilian Society of Plastic Surgery, São Paulo, SP, Brazil
Correspondence to:
Sérgio Aguinaldo de Almeida
Av. Francisco de Paula Quintaninha Ribeiro, 280 - ap.134 - bloco 1
Jabaquara - São Paulo, SP, Brazil -CEP 04330-020
E-mail: estomaterapeuta@outlook.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: December 13, 2012
Article accepted: March 17, 2013
This study was performed at the Plastic Surgery Clinic, São Paulo Hospital - Wound Ward, São Paulo, SP, Brazil, and Wound Clinic of the Sorocaba Hospital, Sorocaba, São Paulo, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter