

Original Article - Year 2013 - Volume 28 -
Merkel cell carcinoma: clinical presentation, prognostic factors, treatment and survival in 32 patients
Carcinoma de células de Merkel: apresentação clínica, fatores prognósticos, tratamento e sobrevida de 32 pacientes
ABSTRACT
BACKGROUND: Merkel cell carcinoma is a rare, aggressive, malignant primary cutaneous neuroendocrine tumor. The objective of this study was to evaluate the epidemiological profile of patients with Merkel cell carcinoma, the clinical characteristics of the tumor, time between manifestation of signs and symptoms and initiation of treatment, survival and causes of death.
METHODS: Thirty-two patients with Merkel cell carcinoma were evaluated retrospectively. Clinical history and staging were correlated with 1 and 2-year survival.
RESULTS: Most patients (69%) were female, mean age was 72 years and 93% were fair-skinned. The most commonly affected sites were the head/neck, trunk and limbs. Other malignancies were found in 6 patients. Mean time between the appearance of signs/symptoms and initiation of specialist treatment was 12.2 months, with regional lymph nodes being affected at that time in 13 (40%) cases and distant metastases being present in 4 (12%). Following specialist treatment, 1 and 2-year survival was 53% and 47%, respectively. Tumor size < 2 cm was indicative of more favorable prognosis.
CONCLUSIONS: Late diagnosis contributed to high lethality due to rapid local and distant progression of the disease.
Keywords: Neuroendocrine carcinoma. Merkel cell carcinoma. Prognosis.
RESUMO
INTRODUÇÃO: O carcinoma de células de Merkel é uma rara neoplasia cutânea primária neuroendócrina agressiva. O objetivo deste estudo foi avaliar o perfil epidemiológico dos pacientes acometidos com carcinoma de células de Merkel, as características clínicas da neoplasia, o tempo até o início do tratamento, a sobrevida e as causas de morte.
MÉTODO: Foram avaliados, retrospectivamente, 32 pacientes portadores de carcinoma de células de Merkel. A história clínica e o estadiamento dos pacientes foram correlacionados em 1 ano e 2 anos de sobrevida.
RESULTADOS: A maioria dos pacientes (69%) era do sexo feminino, com média de idade de 72 anos e 93% de pele clara. A localização mais acometida era cabeça e pescoço, seguida de tronco e membros. Outras neoplasias foram encontradas em 6 pacientes. O tempo médio entre o surgimento dos sinais/sintomas e o tratamento especializado foi de 12,2 meses, com acometimento de linfonodos regionais em 13 (40%) pacientes e metástases à distância em 4 (12%). Após o tratamento especializado, observou-se sobrevida em 1 ano de 53% e em 2 anos, de 47%. Tumor < 2 cm foi indicativo de melhor prognóstico.
CONCLUSÕES: O diagnóstico tardio contribuiu para a alta taxa de letalidade da doença, em decorrência da rápida progressão local e à distância.
Palavras-chave: Carcinoma neuroendócrino. Carcinoma de célula de Merkel. Prognóstico.
Merkel cell carcinoma (MCC) is a rare, primary neuroendocrine carcinoma of the skin1.
The cause of MCC is unknown; however, the disease predominantly affects areas exposed to solar radiation2. This carcinoma is characterized by a high incidence of local recurrence (12%-50%), involvement of the locoregional lymph nodes (17%-76%), distant metastases, and a high lethality rate (20%-55%)3-9.
Therefore, a combination of wide excision of the primary tumor, regional lymphadenectomy and adjuvant radiotherapy has been proposed as the optimal treatment for control of this disease10; however, 1, 2 and 3-year survival rates have been estimated at 88%, 72% and 55%, respectively11.
The objective of this study was to evaluate the epidemiological profile of patients with MCC, the clinical characteristics of the tumor, time between manifestation of signs and symptoms and initiation of treatment, survival and causes of death.
METHODS
A retrospective study was performed of 32 patients with a diagnosis of MCC who had been receiving care at the Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA) between 2002 and 2012; IRB approval was not required due to the retrospective nature of this study.
The epidemiological profile of the patients was established (gender, age and any associated skin neoplasms), staging, topographical distribution, the initial treatment implemented, the time between the appearance of the signs/symptoms and the initiation of specialist treatment, types of postoperative complications, progression with respect to local and distant recurrence, and 1 and 2-year survival rates.
For the purposes of this study, specialist treatment was defined as the therapy implemented following confirmation of MCC. This was established after all the biopsy material was reevaluated by the pathology department at INCA using histopathology and immunohistochemistry. Clinical staging was based on the definitions proposed by Allen et al.6 of the Memorial Sloan-Kettering Cancer Center (Table 1).
The term locoregional metastases was defined as describing a situation in which the lymph node chain closest to the primary tumor was affected. All complications occurring during patient follow-up were evaluated and each complication was described.
RESULTS
Thirty-two patients were included in the analysis, 10 (31%) males and 22 (69%) females. Mean age was 72 years, ranging from 18 to 92 years, with a mean age of 67.6 years for the men and 74.2 years for the women. Regarding to skin color, only two patients were not Caucasian, both of these patients being brown-skinned.
Regarding the site of the lesion, in 18 (56%) cases, the MCC was situated on the head or neck: on the face in 12 cases, on the scalp in 3 and on the neck in the remaining 3. In 6 (19%) cases, the lesion was on the upper limbs, while in 7 (22%) cases it was situated on the trunk and in only 1 (3%) case on a lower limb. In one other case (3%), the lesion was situated on the patient's penis (Figure 1).
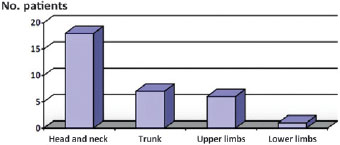
Figure 1 - Topographical distribution of Merkel cell carcinoma: primary tumor.
In 8 (25%) patients, the tumor was < 2 cm in size (T1), while in 24 (75%) cases it was > 2 cm (T2). Mean size was 2.6 cm, ranging from 0.8 to 4 cm.
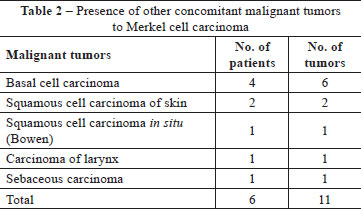
In addition to the MCC, other malignant tumors were found concomitantly in 6 (18.7%) patients. One patient had a basal cell carcinoma (BCC) and a sebaceous carcinoma on the same forearm. Another had a squamous cell carcinoma (SCC) of the larynx. One patient had a SCC in situ (Bowen's disease) on her left forearm and a BCC on her right forearm. One patient had a SCC on his leg and a BCC on his hand. One had a SCC on his scalp and another had a multifocal BCC on his face (Table 2).
The initial forms of treatment implemented were incisional biopsy for the purpose of diagnosis or curative resection. According to histopathology, the margins were affected in 8 of these cases, with the patient dying within less than two years in 6 cases due to progression of the disease. Two patients are still being followed-up on an outpatient basis after 1 and 3 years, respectively, apparently free of the disease.
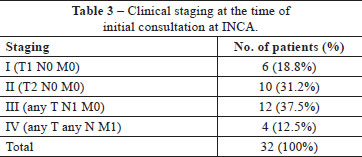
The reasons for the referral of the 32 patients to INCA were: a histopathological diagnosis of neuroendocrine carcinoma; cases in which the disease was refractory to the initial treatment used and/or cases of advanced disease. The mean time between the appearance of the signs/symptoms and the initiation of specialist treatment was 12.2 months. At this time, the regional lymph nodes were found to be affected (N1) in 13 (37.5%) patients, with distant metastases present (M1) in 4 (12.5%) patients (Table 3).
In 9 cases, the first histopathology report was inconclusive or discordant with the definitive diagnosis (defined by immunohistochemistry). Rather than MCC, the initial diagnosis was: undifferentiated carcinoma in 5 cases, cutaneous lymphoma in 3 cases and basal cell carcinoma (BCC) in one case.
Following surgical resection, the incidence of local recurrence was 37% (12 cases). Involvement of the lymph node chain closest to the primary tumor in the postoperative period was also found in 12 (37%) cases. Distant metastases were found in 8 (25%) cases. Of these 8 patients, 3 had diffused subcutaneous dissemination, 2 had intracranial disease, there was pleural involvement in one case, and one patient had a lesion in the lung and one in the liver. All the patients were referred for evaluation with a view to possible adjuvant chemotherapy (CT) and/or radiotherapy (RXT); however, only 9 patients concluded treatment. Six patients died within 1 year.
The postoperative complications consisted of: one case of deep vein thrombosis (DVT), one case of DVT associated with pulmonary thromboembolism (PTE), one patient with pleural effusion followed by respiratory failure, 2 patients with neural invasion (paralysis of the facial nerve and marginal mandibular branch) and 2 patients with profuse bleeding at the site of recurrence.
One-year survival was 53% (17 patients) and 2-year survival was 47% (15 patients). Of these, 6 patients continue to be followed up as outpatients after periods of more than 5 years.
DISCUSSION
In many aspects, the findings of the present study are similar to reports published in the literature. The epidemiological profile of the patient affected by MCC was of an elderly (mean age 72 years), fair-skinned individual. Nevertheless, a finding that differed from the data found in the literature concerned gender, with a predominance of female patients in this present sample12.
With respect to the site of the lesions, the appearance of MCC has long been correlated with exposure to ultraviolet rays13-15. The findings of this study are in agreement with previously published data on this subject, since MCC was located in exposed areas of the body (head, neck and upper limbs) in 24 patients, 75% of all cases. The lesions on the upper limbs of all six patients were limited to the forearm, the anatomical site most exposed to the sun.
There was concomitance with other skin malignancies in 6 patients, with a total of 11 lesions: BCC, SCC and sebaceous carcinoma, all also located on areas exposed to sunlight with the exception of the SCC of the larynx. No cases of melanoma or synchronous MCC were found, although such cases have already been described16.
Another aggravating factor that contributes to unfavorable disease progression was inadequate initial treatment. Eight patients were submitted to noncurative resection of the tumor, a fact confirmed in the histopathology report showing that the surgical margins in the excision specimen were affected. Consequently, with the progression of the disease, 6 patients (75% of this subgroup) died in less than two years.
In addition to these cases, histology reports were inaccurate or inconclusive in nine cases. In three, the patients were referred for chemotherapy and radiotherapy with a diagnosis of cutaneous lymphoma. In another case, the patient was wrongly considered to have been treated, since the initial report was compatible with a BCC. The correct diagnosis was obtained only later when immunohistochemistry was performed. Of these 9 patients, 6 received only palliative treatment, and all died within a short time.
With respect to recurrence, data in the literature on the local aggressiveness of the tumor are in agreement with the findings of the present study in which there was involvement of the lymph node chain closest to the tumor in 12 (37%) patients and local recurrence in 12 (37%) cases (Figure 2). It is important to emphasize that, of the 12 patients with local recurrence, the margins were affected in 8 cases and 4 patients were initially treated as having a skin lesion other than MCC. Surgery was repeated with success in only one patient, who still remains disease-free after the width of the margins was increased.
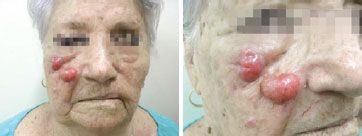
Figure 2 - Female patient, 86 years old, underwent to resection of a skin lesion in right malar region and reconstruction with autograft skin. The diagnosis of Merkel cell carcinoma was obtained only after the initial treatment. Experienced local recurrence and submandibular lymph node on the right, 1 year after the procedure.
Distant aggressiveness was also a factor indicative of poorer prognosis. Distant metastases occurred during or following treatment in 32% of cases. Of these 8 patients, 3 had diffused cutaneous dissemination, even at sites distant from the site of the primary tumor. This diffuse recurrence in the skin and in the subcutaneous cell tissue has been described as being more common in immunocompromised patients16, a fact that was found in one of these cases in which the MCC occurred in a patient with acquired immunodeficiency. The disease progressed rapidly and the patient died from hemorrhagic shock caused by bleeding from a tumor in the gluteal region in addition to his general debilitated state.
Other factors that contributed to a lethal outcome were: deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) in two patients. Both had been submitted to inguinal lymphadenectomy. In one case, the primary tumor was on the penis and in the other case the lesion was on the lower part of the trunk.
It is therefore important to note that the time between the initial appearance of signs and/or symptoms and the beginning of specialist treatment is a factor that is crucial to the prognosis of the disease. Some authors have associated unfavorable clinical progression of the disease with the size of the lesion, showing better results in the treatment in patients with tumors < 2 cm in size (T1)17,18. Indeed, according to the present findings in which there was a mean delay of one year prior to initiating specialist treatment, the presence of lymph node involvement and distant metastases were also found to be factors indicative of poor prognosis in addition to tumor size > 2 cm (T2) (Figure 3).
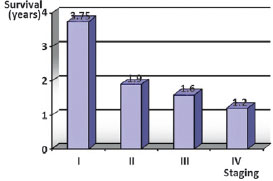
Figure 3 - Relationship of survival in years, according to staging at the beginning of specialized treatment.
Compared to survival data published in the literature3-11, the present results revealed a worse scenario. Of the 32 patients evaluated, only 53% remained alive after one year and only 47% were still undergoing outpatient follow-up at two years. This discrepancy is associated with the factors discussed previously including inadequate initial management, a delay in initiating specialist treatment and the aggressiveness of the disease.
CONCLUSIONS
According to this study, MCC is more common in females, in fair-skinned individuals and in those of around 72 years of age. It affects areas exposed to sunlight such as the upper limbs, head and neck. It may be associated with other malignant tumors, particularly with skin cancer. The factor most significantly associated with favorable prognosis is tumor size < 2 cm with no lymph node involvement or metastases to other tissues or organs. However, delayed diagnosis and a consequent delay in initiating definitive treatment contributes to the high lethality rate associated with this disease, since Merkel cell carcinoma is an aggressively malignant tumor with rapid local and distant progression.
REFERENCES
1. Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. A clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9(2):95-108.
2. Verola O, Champeau F. Merkel cell carcinoma. Ann Chir Plast Esthet. 1998;43(4):439-44.
3. Pilotti S, Rilke F, Bartoli C, Grisotti A. Clinicopathologic correlations of cutaneous neuroendocrine Merkel cell carcinoma. J Clin Oncol. 1988;6(12):1863-73.
4. Eftekhari F, Wallace S, Silva EG, Lenzi R. Merkel cell carcinoma of the skin: imaging and clinical features in 93 cases. Br J Radiol. 1996;69(819):226-33.
5. Ott MJ, Tanabe KK, Gadd MA, Stark P, Smith BL, Finkelstein DM, et al. Multimodality management of Merkel cell carcinoma. Arch Surg. 1999;134(4):388-92.
6. Allen PJ, Zhang ZF, Coit DG. Surgical management of Merkel cell carcinoma. Ann Surg. 1999;229(1):97-105.
7. Nathu RM, Mendenhall WM, Parsons JT. Merkel cell carcinoma of the skin. Radiat Oncol Investig. 1998;6(5):233-9.
8. Victor NS, Morton B, Smith JW. Merkel cell cancer: is prophylactic lymph node dissection indicated? Am Surg. 1996;62(11):879-82.
9. Savage P, Constenla D, Fisher C, Thomas JM, Gore ME. The natural history and management of Merkel cell carcinoma of the skin: a review of 22 patients treated at the Royal Marsden Hospital. Clin Oncol (R Coll Radiol). 1997;9(3):164-7.
10. Kokoska ER, Kokoska MS, Collins BT, Stapleton DR, Wade TP. Early aggressive treatment for Merkel cell carcinoma improves outcome. Am J Surg. 1997;174(6):688-93.
11. Hitchcock CL, Bland KI, Laney RG 3rd, Franzini D, Harris B, Copeland EM 3rd . Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann Surg. 1988;207(2):201-7.
12. Reichgelt BA, Visser O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population-based study of 808 cases in 1993-2007. Eur J Cancer. 2011;47(4):579-85.
13. Mott RT, Smoller BR, Morgan MB. Merkel cell carcinoma: a clinicopathologic study with prognostic implications. J Cutan Pathol. 2004;31(3):217-23.
14. Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49(5):832-41.
15. Satter EK, Derienzo DP. Synchronous onset of multiple cutaneous neuroendocrine (Merkel cell) carcinomas localized to the scalp. J Cutan Pathol. 2008;35(7):685-91.
16. Calza L, Beltrami C, Manfredi R, Colangeli V, Freo E, Chiodo F. Merkel cell carcinoma in a human immunodeficiency virus-infected patient. Br J Dermatol. 2002;146(5):895-8.
17. Mello DF, Felix LRM, Rodrigues A, Helene Jr A. Carcinoma das células de Merkel: relato de 2 casos. Rev Bras Cir Plást. 2010;25(1):217-21.
18. Koljonen V, Böhling T, Granhroth G, Tukiainen E. Merkel cell carcinoma: a clinicopathological study of 34 patients. Eur J Surg Oncol. 2003;29(7):607-10.
1. Resident physician at the do Instituto Nacional de Câncer José Alencar Gomes da Silva (National Cancer Institute José Alencar Gomes da Silva - INCA), Rio de Janeiro, RJ, Brazil
2. Plastic surgeon, full member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), master at Universidade Estadual de Campinas (State University of Campinas - Unicamp), preceptor of the Plastic Surgery Service of the INCA, Rio de Janeiro, RJ, Brazil
3. Plastic surgeon, full member of the SBCP, Head of the Plastic Surgery and Reconstructive Microsurgery Service of the INCA, Rio de Janeiro, RJ, Brazil
Correspondence to:
Coracy Carneiro
Rua Alto Santo, 82 - José Bonifácio
Fortaleza, CE, Brazil - CEP 60055-340
E-mail: fco_coracy@yahoo.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: February 2, 2013
Article accepted: June 29, 2013
This study was performed at the Plastic Surgery Service of the Cirurgia do Instituto Nacional de Câncer José Alencar Gomes da Silva (National Cancer Institute José Alencar Gomes da Silva), Rio de Janeiro, RJ, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter