

Original Article - Year 2013 - Volume 28 -
Late seroma after silicone breast implants: three different forms of presentation, evolution, and approach
Seroma tardio após implantes mamários de silicone: três formas diferentes de apresentação, evolução e conduta
ABSTRACT
BACKGROUND: Late seromas after augmentation mammaplasty are uncommon, can manifest without a defined cause, and can be treated by implant removal or replacement. This study aimed to analyze three cases of this complication that occurred 1-10 years postoperatively and were treated differently.
METHODS: Data of three patients who developed late seroma after breast implant placement were analyzed. In this report, we present data on the indication for implant placement, time without complications, implant type, and the analytical results of the removed or drained material.
RESULTS: Two patients underwent bilateral implant removal, although only one side was affected, and the implant was replaced with another of the same type and volume in the third patient. One case of sterile pus was diagnosed.
CONCLUSIONS: Before undergoing breast implant surgery, patients should be informed of the implications of their decisions, such as the possible need to remove or replace them, resulting in more surgical procedures and/or new scars.
Keywords: Breast implantation/adverse effects. Seroma. Breast/surgery. Mammaplasty.
RESUMO
INTRODUÇÃO: Os seromas tardios após mamoplastia de aumento são ocorrências pouco comuns, que podem se manifestar sem causa definida e cujo tratamento implica a retirada ou a troca das próteses. Este trabalho objetiva analisar 3 casos dessa complicação, ocorrida entre 1 ano e 10 anos de pós-operatório, tratados de formas distintas.
MÉTODO: Foram analisados os dados de 3 pacientes que apresentaram seroma no pós-operatório tardio de inclusão de próteses de mama. Neste artigo são apresentados dados relativos a indicação da inclusão, tempo de evolução sem complicações, tipo de prótese e resultado da análise do material retirado ou drenado.
RESULTADOS: Em 2 pacientes, foi realizada retirada bilateral das próteses, apesar de somente um dos lados ter sido afetado; na terceira paciente, procedeu-se à troca da prótese por outra de mesmo volume e tipo. Um dos casos foi diagnosticado como pus estéril.
CONCLUSÕES: Previamente à inclusão de próteses mamárias, a paciente deve ser alertada para o fato de que sua decisão poderá ter implicações futuras, sendo, eventualmente, necessário trocá-las ou retirá-las, o que resultará em novas cirurgias e/ou novas cicatrizes.
Palavras-chave: Implante mamário/efeitos adversos. Seroma. Mama/cirurgia. Mamoplastia.
Over the past few decades, augmentation mammaplasty has been increasingly performed in Brazil and worldwide with increasingly better and safer results. However, the procedure involves the use of a foreign body and the risk of complications distinct from those associated with other types of surgery. Cunningham et al.1 indicated that the most common recently noted complications include hypertrophic scars, hematomas, seromas, dehiscence, and infection. Late complications include asymmetry, changes in contour, contractures, hyper- or hyposensitivity, deflation (in saline implants), and rupture.
Seromas are unusual late complications that can manifest without a defined cause, and whose treatment involves implant removal or replacement. If the etiology is undefined, there is no effective prophylaxis; therefore, patients should be informed about the possibility of adverse mid- or long-term postoperative events.
This study aimed to examine three patients who developed this complication 1-10 years postoperatively and who were treated differently.
METHODS
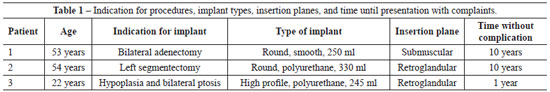
We analyzed the data of the three patients aged 53, 54, and 22 years who underwent breast augmentation and developed seroma during the late postoperative period. None of the patients had associated previous illnesses or allergies. The indication for breast implant placement was breast parenchymal resection or glandular hypoplasia. Implant type and volume, the insertion plane, and the duration between surgery and presentation with complaints are shown in Table 1.
RESULTS
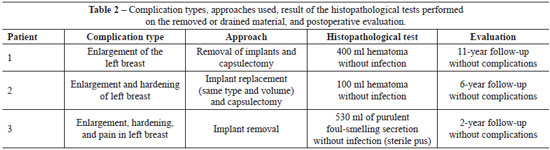
Table 2 shows the complication types, approaches used, results of the histopathology tests performed on the removed or drained material, and the postoperative follow-up duration.
Two patients underwent bilateral implant removal although only one side was affected; in the third patient, the implant was replaced with another implant of the same type and volume. These three patients presented with no complications during the immediate postoperative follow-up period. Figures 1 to 3 show the reported cases.
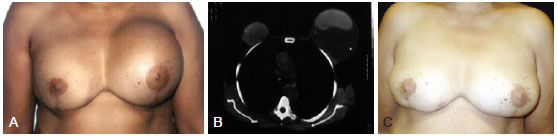
Figure 1 - A 53-year-old patient underwent bilateral adenectomy 10 years previously for fibrocystic disease and placement of smooth, round, 250 ml silicone implants in the submuscular plane. In A, left breast enlargement. In B, computerized tomography. In C, late postoperative appearance (11 years) with only modeling of local tissue.
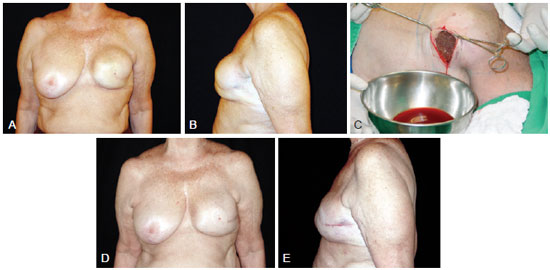
Figure 2 - A 54-year-old patient who underwent left segmentectomy 10 years ago for breast cancer and the placement of a round, polyurethane-covered 330 ml implant in the retroglandular plane. In A and B, left breast enlargement (frontal view and left-facing profile, respectively). In C, appearance of the drained serosanguineous fluid. In D and E, postoperative appearance 6 years after replacement of the breast implant during the same surgical procedure (frontal view and left-facing profile, respectively).
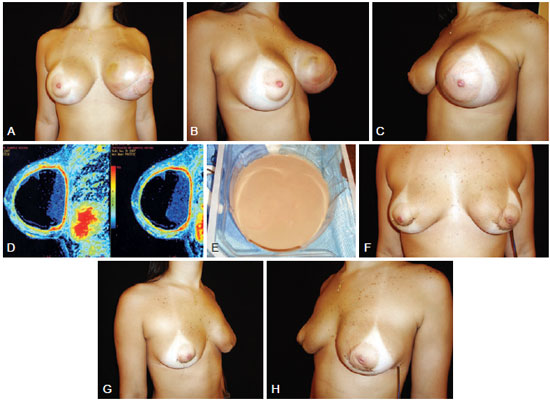
Figure 3 - A 22-year-old patient underwent placement of high-profile 245 ml polyurethane implants 1 year previously in the retroglandular plane for esthetic purposes. In A, B and C, breast enlargement (frontal view, right-sided profile, and left-sided profile, respectively). In D, magnetic resonance image. In E, appearance of the collected fluid (sterile pus). In F, G, and H, appearance on day 4 after removal of both implants (frontal view, right-sided profile, and left-sided profile, respectively).
DISCUSSION
Various early or late complications can occur after breast implantation. Fluid, serum, blood, or purulent collections occur mostly frequently in the early postoperative stage. Hematomas and infections should be treated immediately, with or without implant removal. Fluid collection (seromas), if minor, is quite common and not always symptomatic. Ahn et al.2 found intracapsular fluid in 21% of patients who underwent implant replacement for various reasons. Even large amounts of accumulated fluid (with no associated complications) can be removed by puncture with ultrasound guidance.
The incidence of infection varies among different studies; we have not encountered cases of infection. Netscher et al.3 observed positive cultures in 27.3% of patients whose implants were removed for other reasons and who did not exhibit clinical signs of infection; the most frequent infectious agents were coagulase-negative staphylococci and anaerobic diphtheroids. Treatment strategies vary from simple antibiotic therapy to implant removal.
In 1999, Holmich et al.4 randomly selected 271 women with breast implants who were followed up for at least 3 years postoperatively and examined them using magnetic resonance imaging. In 2001, they repeated the exam and found 33 (12.2%) confirmed ruptures and 23 (8.5%) suspected ruptures. These authors observed that the incidence of rupture increases with implant age; at least 15% of implants rupture 3-10 years after surgery.
Brandon et al.5 stated that implant rupture can occur before or during insertion, during removal, or in situ. Although the natural resistance of implants and their durability has been widely discussed, the authors believe that some ruptures occur during pre- or perioperative implant handling, insertion, or removal by accidental contact with surgical instruments or abrasion of the implant surface when it passes through very small incisions. In the case of cohesive silicone implants, small degrees of damage can go unnoticed due to the inherent characteristic of this type of silicone, which exhibits minimal leakage. The authors produced different degrees of intentional damage in laboratory implants and used electron microscopy to determine the rupture type produced by each type of instrument. A better understanding of the mechanisms that lead to rupture, other than elastomer degradation, contributes to the production of better designed and more durable implants.
Late seromas after breast augmentation using implants are rare and can be a sign of implant rupture, although the limited number of reported cases does not allow categorical conclusions6. Serum and serosanguineous collections are the most frequent findings. Sterile pus is very rarely observed and occurred in one case in the present study.
Late hematoma was first reported in 1979 by Georgiade et al.7, who identified the complication 2.5 years after the inclusion of smooth 185 ml saline silicone implants. During surgical exploration, 300 ml of blood was collected from an actively bleeding artery. The culture was sterile. The authors attributed the cause of bleeding to the use of triamcinolone to prevent capsular contracture during implant insertion.
Gorgu et al.8 stated that late hematomas are rare (according to the authors, four cases had been described until the publication of the study in 1999) and reported one case of rupture that occurred 3 years after insertion of a textured saline-filled implant in the subpectoral plane using intracapsular corticoid. The authors attributed the cause of bleeding to the use of corticoids as well as abrasion between the implant and fibrous capsule.
Brickman et al.9 reported one case of hematoma that occurred 9 years after the insertion of 385 ml polyurethane implants. They attributed the pathogenesis of the late hematomas to prolonged inflammatory reactions with increases in capillary permeability (Labardie and Glover theory of subdural hematomas), which were supposedly worse in polyurethane-covered implants. However, these hematomas have also been reported in smooth or textured implants8,10.
Roman & Perkins11 explored a few theories to explain late hematomas and suggested that they could result from microfractures in rigid capsules that cause bleeding. Fluid accumulation could also be related to an osmotic effect secondary to an inflammatory process. They also reported one case of hematoma that developed 22 years after breast implant insertion.
Pinchuk & Tymofii12 reported six cases of late seroma that occurred 2-10 years postoperatively in a sample of 568 patients who underwent breast augmentation with implants. According to the authors, abrasion between the implant and the internal surface of the organic capsule contributed to the synovial metaplasia observed in this surface as well as to chronic infection and subsequent seroma formation.
Robinson et al.13 observed 14 cases of spontaneous enlargement in 3,000 patients with saline implants. Fluid cultures were all negative. The precise mechanism causing this increase in volume is not completely understood, especially because it can occur on only one side. Using bidimensional electrophoresis and mass spectrometry, these authors found four known proteins in the fluid, with serum albumin being predominant. They stated that spontaneous enlargement is probably an underreported occurrence.
Peters14, who also investigated saline implant enlargement, discussed the possible existence of osmotic gradients between the body and the fluid used to fill the implants; however, the author considered that the probable cause of the event was the mechanical changes in the valves.
Unlike in silicone implants, the increase in volume in the saline implants occurs inside the implant and not around it. A correlation does not appear to exist between enlargement caused by increases in intraimplant volume and enlargement caused by seroma forming around the silicone gel implants.
Khan6 reported five consecutive cases of spontaneous enlargement caused by sterile pus accumulation 2-10 years after silicone (two cases) or hydrogel (two cases) implant insertion. In all cases, the collected fluid was sterile. Four implants ruptured and the fifth implant showed a microscopic rupture. All patients underwent single-stage surgery in which the pockets were cleaned, capsules were removed, implants were replaced, and the pockets were moved to the submuscular plane. There were no complications and all patients had satisfactory outcomes.
In the three cases reported in the present study, the fluid was sterile; however, the fluid characteristics differed among the cases: blood clumps and clots were seen in the first patient, serosanguineous fluid was seen in the second patient, and sterile pus was seen in the third patient. Moreover, different types of implants were used: smooth implants in the first case and polyurethane-covered implants in the other two cases. The literature does not describe a causal relationship between late seroma and implant size, coverage type, access route, or insertion plane. It is worth noting that late hematomas, although uncommon, are more frequent9,10,15 than sterile pus collections, which are very rare6.
Spear et al.16 studied 24 patients with 26 implants affected by infection or exposure. The implant was salvaged in 94.7% of patients with no or mild infection (18 of 19 patients); in cases of severe infection, this percentage dropped to 28.5% (two of seven patients). They called it "salvage the implant" when the patient had an implant after surgery (regardless of original or replacement). Their methods included systemic antibiotic therapy, curettage, capsulectomy, and change of pocket or implant. Although attempts to salvage the implants were valid, the authors considered implant removal and subsequent reinsertion optimal.
The need to remove implants, in particular those that are placed for esthetic reasons, is always a difficult decision for the surgeon and involves emotional trauma for the patient. Knowing when to remove an implant and how to manage patients who go experience such frustration are skills required by all plastic surgeons. The conservative approach is always the first choice. When the option was to intervene (and there were no signs of infection), some authors decided to replace the implants after capsule removal6,16. This was the approach used in the second case reported in the present study. In the first case, the patient opted for implant removal and modeling using the remaining tissue15.
However, in the third case reported in the present study, the evolution was dramatic, symptoms were intense, and the patient understood that the solution to the problem would depend on implant removal. This psychological change helped the patient's immediate emotional state. Khan6, whose studies also analyzed enlargement in the presence of sterile pus, referred to having replaced implants after capsulotomy, thus resolving the problem in a single stage. Although he reported a successful outcome, this approach seems unwise considering the fluid's characteristics because the culture results are not immediately available, and even if there are no symptoms of infection, the fluid's purulent appearance is troubling. In the case of our patient, there was an undetermined foul smell. We preferred to postpone insertion of the new implant for at least 6 months to the point at which the patient was willing to undergo the procedure.
There is always doubt about what to do with the contralateral side with the normal implant. We believe that removal is the best approach because the resulting asymmetry would be difficult to resolve and will always remind the patient of the complication. However, all of the implications of this decision, such as an unsatisfactory shape (in breasts that had implants that were not replaced) and the presence of scars (if one decides to model the existing tissue) needs to be discussed with the patient. In the first case reported in the present study, the patient did not want new implants and the modeling resulted in a satisfactory shape because of the abundant local tissue15. In the second case, a small amount of clear fluid was collected without signs of infection, which allowed immediate implant replacement. In the third case, removal of the 245 ml implants did not cause any deformity and the breasts' appearance was similar to that before implant insertion (likely due to the patient's young age).
Late seromas after breast implants have developed after both silicone6,9,15 and saline implant placement7,13,14. In silicone implants, the fluid accumulates outside the implant. Assumptions about the etiology suggest implant rupture, abrasion between the implant and the organic pocket, the chronic inflammatory process, and metaplasia of the internal surface of the capsule. In saline implants, the expansion occurs inside the implant, possibly due to the presence of an osmotic gradient between the patient's body fluids and the fluid used to fill the implant.
CONCLUSIONS
Before undergoing breast implant surgery, patients should be informed that their decision may have future implications such as the possible need for implant replacement or removal, which means more surgical procedures and/or more scars. The gratifying results of this surgery are worth the small risk; however, the provision of adequate information and informed consent are essential for patients' emotional well-being in the event of complications.
REFERENCES
1. Cunnigham BL, Lokeh A, Gutowski KA. Saline-filled breast implant safety and efficacy: a multicenter retrospective review. Plast Reconstr Surg. 2000;105(6):2143-9.
2. Ahn CY, Ko CY, Wagar EA, Wong RS, Shaw WW. Clinical significance of intracapsular fluid in patients' breast implants. Ann Plast Surg. 1995;35(5):455-7.
3. Netscher DT, Weizer G, Wigoda P, Walker LE, Thornby J, Bowen D. Clinical relevance of positive breast periprosthetic cultures without overt infection. Plast Reconstr Surg. 1995;96(5):1125-9.
4. Holmich LR, Friis S, Fryzek J, Vejborg IM, Conrad C, Sletting S, et al. Incidence of silicone breast implant rupture. Arch Surg. 2003;138(7):801-6.
5. Brandon HJ, Young VL, Jerina KL, Wolf CJ. Scanning electron microscopy characterization of surgical instrument damage to breast implants. Plast Reconstr Surg. 2001;108(1):52-61.
6. Khan UD. Breast autoinflation with sterile pus as a marker of implant rupture: single-stage treatment and outcome for five consecutive cases. Aesthetic Plast Surg. 2009;33(1):58-65.
7. Georgiade NG, Serafin D, Barwick W. Late development of hematoma around a breast implant, necessitating removal. Plast Reconstr Surg. 1979;64(5):708-10.
8. Gorgu M, Aslan G, Tuncel A, Erdogan B. Late and long-standing capsular hematoma after aesthetic breast augmentation with a saline-filled silicone prosthesis: a case report. Aesthetic Plast Surg. 1999;23(6):443-4.
9. Brickman M, Parsa NN, Parsa FD. Late hematoma after breast implantation. Aesthetic Plast Surg. 2004;28(2):80-2.
10. Veiga DF, Veiga Filho J, Schnaider CS, Archangelo I Jr. Late hematoma after aesthetic breast augmentation with textured silicone prosthesis: a case report. Aesthetic Plast Surg. 2005;29(5):431-3.
11. Roman S, Perkins D. Progressive spontaneous unilateral enlargement of the breast twenty-two years after prosthetic breast augmentation. Br J Plast Surg. 2005;58(1):88-91.
12. Pinchuk V, Tymofii O. Seroma as a late complication after breast augmentation. Aesthetic Plast Surg. 2011;35(3):303-14.
13. Robinson OG Jr, Benos DJ, Mazzochi C. Spontaneous autoinflation of saline mammary implants: further studies. Aesthet Surg J. 2005;25(6):582-6.
14. Peters W. Autoinflation of saline-filled inflatable breast implants. Can J Plast Surg. 2006;14(4):219-26.
15. Franco D, Medeiros J, Destefani V, Franco T. Hematoma tardio após reconstrução de mama com prótese de silicone. Rev Soc Bras Cir Plást. 2006;21(4):227-30.
16. Spear SL, Howard MA, Boehmler JH, Ducic I, Low M, Abbruzzesse MR. The infected or exposed implant: management and treatment strategies. Plast Reconstr Surg. 2004;113(6):1634-44.
1. Plastic surgeon, full member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), member of the National Academy of Medicine, master and doctor in Plastic Surgery, full professor of Plastic Surgery at the Universidade Federal do Rio de Janeiro (Federal University of Rio de Janeiro - UFRJ), Rio de Janeiro, RJ, Brazil
2. Plastic surgeon, full member of the SBCP, master and doctor in Plastic Surgery by the UFRJ, adjunct professor at the UFRJ, Rio de Janeiro, RJ, Brazil
Correspondence to:
Talita Franco
Rua Ramon Franco, 98 - Urca
Rio de Janeiro, RJ, Brazil - CEP 22250-040
E-mail: talita@openlink.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: October 24, 2012
Article accepted: March 1, 2013
This study was performed at the Universidade Federal do Rio de Janeiro (Federal University of Rio de Janeiro - UFRJ), Rio de Janeiro, RJ, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter