

Original Article - Year 2013 - Volume 28 -
Radical versus conservative surgery for craniofacial fibrous dysplasia: stratification of surgical strategies
Cirurgia radical versus cirurgia conservadora no tratamento da displasia fibrosa craniofacial: estratificação da abordagem cirúrgica
ABSTRACT
BACKGROUND: To date, there is no consensus regarding the best surgical approach (conservative or radical) for craniofacial fibrous dysplasia. This study presented the experience of a single institution in the surgical treatment of craniofacial fibrous dysplasia.
METHOD: This was a retrospective analysis of patients with craniofacial fibrous dysplasia who underwent surgery between 1997 and 2012. Surgical treatment was individualized according to patient age, the involved anatomical site (zones I-IV), aesthetic and/or functional impairment, and the preferences of the patients and surgical team. The surgical results were classified on the basis of the Whitaker system.
RESULTS: Ten, 1, 1, and 1 patients with zone I, zone II, zone I/II, and zone I/IV involvement, respectively, were included in the study. In total, conservative surgeries and 9 radical surgeries were performed for the treatment of primary bone tumors. There was 1 surgical complication, and 6 recurrences were identified during the postoperative follow-up period. The global average of surgical outcomes, according to the Whitaker scale, was 1.69 ± 0.94.
CONCLUSIONS: According to the experience and surgical results presented in this study, the surgical approach for craniofacial fibrous dysplasia should be individualized.
Keywords: Fibrous dysplasia of bone. Bone neoplasms. Surgical procedures, operative.
RESUMO
INTRODUÇÃO: Até o momento, não existe consenso sobre qual a melhor abordagem cirúrgica (conservadora ou radical) da displasia fibrosa craniofacial. O objetivo deste estudo foi apresentar a experiência de uma única instituição no tratamento cirúrgico da displasia fibrosa craniofacial.
MÉTODO: Trata-se de uma análise retrospectiva dos pacientes com displasia fibrosa craniofacial, operados entre 1997 e 2012. O tratamento cirúrgico foi individualizado de acordo com idade, sítio anatômico envolvido (zonas I-IV), comprometimento estético e/ou funcional e preferências dos pacientes e da equipe cirúrgica. Os resultados cirúrgicos foram classificados com base no sistema de Whitaker.
RESULTADOS: Dez pacientes com acometimento da zona I, um da zona II, um das zonas I e III, e um das zonas I e IV foram incluídos. Nove cirurgias conservadoras e nove cirurgias radicais foram realizadas para o tratamento de tumores ósseos primários. Houve uma complicação cirúrgica. Seis recidivas foram identificadas durante o seguimento pós-operatório. A média global dos resultados cirúrgicos, de acordo com a escala de Whitaker, foi de 1,69 ± 0,94.
CONCLUSÕES: De acordo com a experiência e resultados cirúrgicos apresentados neste estudo, a abordagem cirúrgica da displasia fibrosa craniofacial deve ser individualizada.
Palavras-chave: Displasia fibrosa óssea. Neoplasias ósseas. Procedimentos cirúrgicos operatórios.
Fibrous dysplasia is a benign bone tumor in which the normal bone structure is replaced by abnormal fibrous bone tissue1,2. This disease is responsible for approximately 2-3% of all tumors derived from bone tissue, and the disease can involve one (monostotic form; represents approximately 70-80% of all cases) or multiple bones (polyostotic form), including the craniofacial skeleton (10-25% of all cases of monostotic fibrous dysplasia and 50-90% of all cases of polyostotic fibrous dysplasia)1,2.
Craniofacial fibrous dysplasia typically appears in childhood, and it is characterized by slow and painless tumor growth causing aesthetic impairment (craniofacial asymmetry) and functional deficits, such as obstruction of the upper airway, dentition disorders, dental and vision occlusion, orbital dystopia, and exophthalmos, depending on the growth of the tumor and the structures involved1,2.
The main goals in the treatment of craniofacial fibrous dysplasia are to correct or prevent functional deficits and restore the aesthetics of the craniofacial contour1,2. Although surgical intervention is considered the primary therapeutic option1,2, there are disagreements concerning the optimal surgical strategy. In addition, therapeutic algorithms can differ3,4. Some craniofacial surgery centers favor large bone resections with immediate bone reconstruction (radical surgical approach)5,6, whereas others favor thinning, curettage, and/or bone modeling (conservative surgical approach)7,8. Moreover, different studies2,3 have reported divergent findings regarding the need for prophylactic orbital decompression and surgical treatment in children.
In 2007, our group9 reported a 2-stage surgical treatment for a form of hereditary familial fibrous dysplasia known as cherubism. However, to our knowledge, there is little national literature that specifically addresses the differences in the surgical treatment of nonhereditary craniofacial fibrous dysplasia10,11, although the demographic, clinical, histopathological, radiographic, and tomographic aspects have been previously characterized10-13.
Thus, this study aimed to present the experience of a Brazilian institution of craniofacial plastic surgery concerning the surgical approach for nonhereditary craniofacial fibrous dysplasia.
METHOD
We performed an observational retrospective study of patients with nonhereditary craniofacial fibrous dysplasia who underwent treatment in the Institute of Craniofacial Plastic Surgery of the SOBRAPAR Hospital between 1997 and 2012. The medical records of all patients with craniofacial fibrous dysplasia were reviewed after approval by the Committee of Ethics and Human Research of the SOBRAPAR Hospital. Only patients with clinical, radiologic, and histologic diagnoses of fibrous bone dysplasia who were treated surgically by the same group of plastic surgeons with similar training and philosophies and who were not lost in the postoperative follow-up were included. Demographic, clinical, and surgical variables were ascertained through medical records, photographs, and clinical consultations with all included patients.
Patients were classified according to the level of involvement of the craniofacial skeleton by fibrous dysplasia, as described by Chen & Noordhoff14, as follows: zone I (fronto-orbital, zygomatic, and upper jaw regions); zone II (scalp); zone III (skull base, including the petrous temporal bone, the pterygoid and mastoid regions, and the sphenoid bone); and zone IV (dentoalveolar maxillary and mandibular regions) (Figure 1).
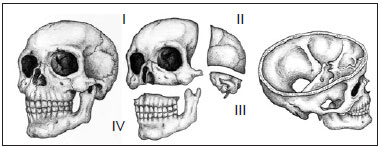
Figure 1 - Schematic representation of the degree of craniofacial skeleton involvement of fibrous dysplasia. (Left) Oblique view of the craniofacial skeleton. (Center) Oblique view of the subdivision of the craniofacial skeleton (upper, left) zone I (fronto-orbital, zygomatic, and upper jaw regions), (upper, right) zone II (bones of the skull), (lower right), zone III (petrous temporal bone, the pterygoid and mastoid regions, and part of the sphenoid bone), and (lower, left) zone IV (maxillary dentoalveolar and mandibular regions) (right). Oblique supracranial view of the craniofacial skeleton showing the skull base (zone III).
Surgical Interventions
All patients underwent conservative (bone thinning) or radical (complete resection of dysplastic bone and immediate bone reconstruction) surgery through an extraoral and/or intraoral approach under general anesthesia to correct functional deficits and improve craniofacial aesthetics when deformities were present and they undermined patients' interpersonal relationships. Decisions about surgical procedures were individualized on the basis of patient age, the anatomical location of the lesion, the presence of orbital dystopia, and the preferences of the patients (or parents when indicated) and surgeons. All patients were regularly followed up after surgery because of the risk of tumor recurrence and malignant potential. The need for new interventions after relapse was based on symptomatology and radiographic disease progression.
The results of the initial/main surgical interventions were graded according to the degree of need for additional surgery described by Whitaker15 as follows: category I, requires no surgical refinements; category II, requires minor surgical refinements of craniofacial contour; category III, requires considerable additional osteotomies (surgical intervention lower than the initial/major surgery); and category IV, requires a new complete craniofacial surgery, similar to the initial/major surgery.
All data were compiled in the Excel for Windows program (Microsoft Corporation, USA). For descriptive analysis, the mean was used for metric variables, and percentages were used for categorical variables.
RESULTS
Thirteen patients diagnosed with nonhereditary craniofacial fibrous dysplasia were included in this study. Seven (53.85%) patients were female, and 6 (46.15%) were male. In 5 (38.46%) patients, fibrous dysplasia was considered congenital (bone disease present from birth), and the mean age at onset of fibrous dysplasia in the remaining 8 (61.54%) patients was 8.75 years (range, 3 months to 25 years). The mean ages of patients at the first craniofacial surgery and at the time of data collection for this study were 16.92 ± 6.92 (range, 7-28 years) and 22.42 ± 8.91 years (range, 9-36 years), respectively.
All patients exhibited progressive asymmetry of their craniofacial contours, which was the main reason they sought specialized care. Ten (76.92%) patients displayed involvement of a single bone (monostotic form), whereas (23.08%) had disease in more than 1 bone (polyostotic form). Ten (76.92%), 1 (7.69%), 1 (7.69%), and 1 (7.69%) patients displayed involvement of zone I, zone II, zones I and III, and zones I and IV, respectively. One (7.69%) patient had McCune-Albright syndrome (polyostotic fibrous dysplasia, precocious puberty, and abnormal skin pigmentation) and other associated congenital abnormalities (cleft palate, macrostomia, unilateral microtia, and preauricular and bilateral malar hypoplasia). Each patient's family history was normal. No malignancy was noted in any of the histopathological analyses of this series.
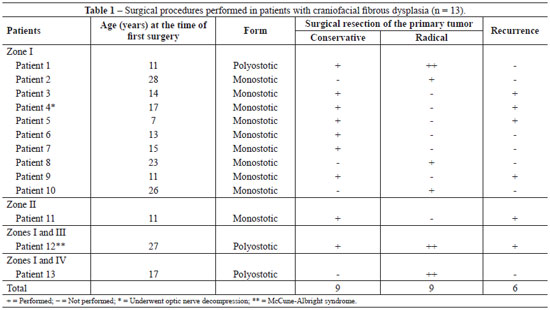
A total of 18 craniofacial surgeries (1-3 surgeries/patient; 9 conservative surgeries and 9 radical surgeries) were performed for the treatment of primary bone tumors according to the severity of aesthetic and/or functional involvement (Figures 2-6). Seven (53.85%) patients exclusively underwent bone thinning (conservative approach), 4 (30.77%) patients underwent extensive bone resection associated with immediate reconstruction of the bone defect (radical intervention), and 2 (15.38%) patients underwent both (radical and conservative) interventions (Table 1).
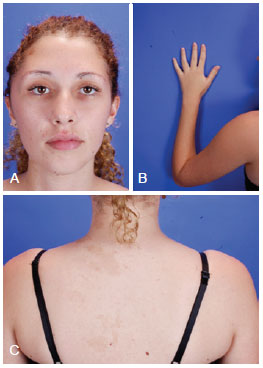
Figure 2 - Patient with McCune-Albright syndrome, aged 27 years. (Upper, left) Frontal photograph revealing zone I involvement of craniofacial fibrous dysplasia. (Upper, right) Photograph showing deformities at the end of the left arm. (Lower) Photograph showing café au lait spots.
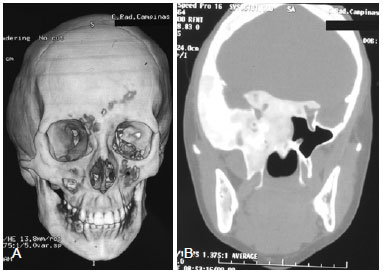
Figure 3 - (Left) Computed tomography (CT) scan with three-dimensional reconstruction of the same patient described in Figure 2 revealing temporal bulging due to increased thickness inherent in the replacement of normal bone by fibrous tissue bone. (Right) Axial CT section demonstrating involvement of the skull base (zone III) with infiltration of the sella turcica, leading to a change of the regulatory mechanism of the pituitary hormone.
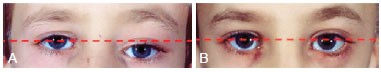
Figure 4 - (Left) Preoperative photograph of the orbital region of a patient with zone I impairment by craniofacial fibrous dysplasia. Note the orbital dystopia (red dash). (Right) Photograph of the orbital region showing the absence of orbital dystopia (rectilinear pattern of red dash) after radical surgery.
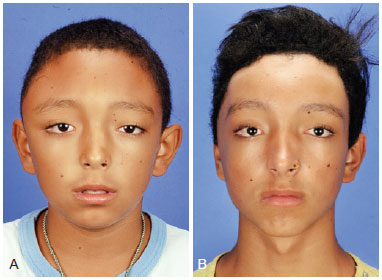
Figure 5 - (Left) Preoperative frontal photograph of a patient with zone I impairment by craniofacial fibrous dysplasia. (Right) Frontal postoperative photograph showing significant improvement of the craniofacial contour after conservative surgery.
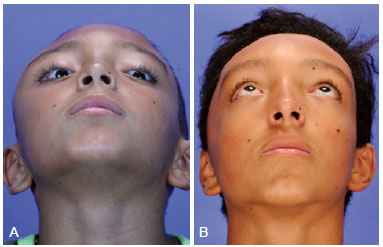
Figure 6 - (Left) Preoperative oblique photograph of the same patient described in Figure 5. (Right) Postoperative oblique photograph showing significant improvement of the craniofacial contour after conservative surgery.
An important improvement in functional and overall craniofacial appearance was obtained in all 13 patients evaluated. The global average surgical result ranked according to the Whitaker scale was 1.69 ± 0.94, with 8 (61.54%), 1 (7.69%), and 4 (30.77%) patients were classified into categories I, II, and III, respectively.
The mean duration of postoperative follow-up was 5.92 ± 3.55 years (range, 1-15 years). Two (15.38%) patients (zone I) experienced visual impairment during the postoperative follow-up period, 1 (7.69%) displayed transient visual loss, and another (7.69%) developed permanent visual loss, even after undergoing decompression craniectomy and decompression of the optic nerve. Six (46.15%) patients experienced a recurrence of fibrous dysplasia at the primarily affected anatomical site during the postoperative follow-up. All recurrences occurred in patients who underwent conservative surgery. No new bone tumors were observed in other anatomical sites during the follow-up period.
There was only 1 (5.56%) complication (surgical site hematoma) after conservative surgery (bone thinning of the frontal region). All patients are being followed at the SOBRAPAR Hospital.
DISCUSSION
Fibrous dysplasia is the most common craniofacial bone tumor observed in plastic surgery1,5. The disease mainly affects women7,8,16, and approximately 1.5-5.40% of cases of craniofacial fibrous dysplasia are associated with McCune-Albright syndrome4,5,16, as noted in our study.
The primary treatment for craniofacial fibrous dysplasia is surgery5,17,18. One must mention that this treatment is associated with controversies, including the need for prophylactic orbital decompression, the best type of surgical approach (conservative or radical), and the use of surgical interventions in children3.
The most dramatic consequence of craniofacial fibrous dysplasia is visual deficit due to compression of the optic nerve (present in 50-90% of patients), and there is a debate concerning the need for prophylactic optic nerve decompression, especially when the vision of the patients is normal2,3-5,19,20. Because of the potential risk of visual impairment and optic nerve atrophy, prophylactic decompression was historically indicated for asymptomatic patients with radiologic evidence of optic nerve compression21. However, a recent meta-analysis20 illustrated that decompression surgery in asymptomatic patients was associated with the deterioration of visual acuity. In fact, narrowing of the optic nerve by bone fibrous dysplasia alone is not related to visual loss, as 95% of patients maintain normal vision despite the extrinsic tumor compression19. Moreover, most recent studies5,20 revealed that approximately 67-84% of patients with visual impairment who underwent decompression surgery displayed improved visual acuity.
Thus, we and other groups4-6 recommend surgical decompression of the optic canal only in patients with orbital involvement and commitment of visual acuity. In this context, Chen et al.21 thus defined the indication for decompression surgery of the optic nerve as follows: gradual and progressive visual loss and sudden visual loss (within 1 week) are considered absolute indications for immediate surgical decompression; rapid visual loss (within 2-3 weeks), no visual loss with radiographic evidence of reduction of the optic canal (because of the progressive growth of bone tumors) in children and adolescents, and no visual loss with radiographic evidence of reduction of the optic canal and with the active fibrous dysplasia in adults are relative indications. In line with other groups5,20,22, we adopted only a few of these guidelines21 in our practice. Asymptomatic patients with computed tomographic evidence of bone around the optic nerve tumor have been regularly monitored with evaluation of visual function5,6,20,22, whereas those who experienced visual deficits for less than 1 month undergo decompression surgery because the intervention appears to be useless in patients who experienced compromised visual acuity for more than 1 month5.
In the present study, the only patient (7.69%) who underwent optic nerve decompression 2 weeks after presenting with unilateral amaurosis developed permanent unilateral visual loss. Other studies5,20 have also reported that visual deficits may persist in patients with visual impairment, even if decompression is performed within 1 month.
In agreement with the present study, most authors4,5,16-18 reported that the relapse of fibrous dysplasia is more frequent in patients treated with a conservative approach (25-80%). Based upon this observation, other authors5,6 recommended radical surgery as the primary surgical treatment modality despite its significantly higher risk of intraoperative bleeding5.
In this context, as the clinical presentation of craniofacial fibrous dysplasia is extensive, depending on the site involved and the tumor extension16, the surgical approach should be selected carefully17. We believe that, in addition to the relevance of recurrence, other aspects such as age, the affected bone site, functional and/or aesthetic impairment, the preferences of patients and family members, and the experience of the surgical team should considered before making any decisions2,7,16. In other words, treatment must be individualized for each particular clinical situation2,4,23.
Historically, it has been recommended to wait for the stabilization of dysplastic bone growth after puberty before surgically treating patients with craniofacial fibrous dysplasia16-18. However, as craniofacial involvement is devastating for both interpersonal relationships and function in children, this delay has been less acceptable16,18. Thus, early surgical intervention is necessary and logical, as bone tumors compromise function and aesthetics in children16. One must mention that within the scope of surgical indication, function is more relevant than aesthetics3,8,18.
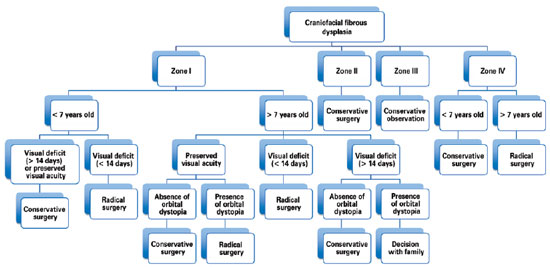
Hence, our group has treated patients with craniofacial fibrous dysplasia under the following philosophy: lesions in the anterior skull base (anterior and middle cranial fossa) are treated with radical surgical intervention, whereas lesions in the posterior skull base (posterior fossa) have been treated with conservative surgery because of the difficulty in achieving complete tumor removal/reconstruction. Lesions of the orbital, zygomatic, maxillary, and mandibular regions (zones I and IV) in patients less than 7 years of age who do not exhibit any visual impairment have been treated conservatively because osteotomy can compromise the development of dentition. As chewing and/or respiratory functions are rarely affected by zone IV impairment, radical surgery can be delayed until craniofacial growth is more established and closer to completion. Therefore, as 85% of the growth of the craniofacial skeleton is complete by 7 years of age24, any radical surgery after this age will have a minor impact on facial growth. The presence of visual deficits for less than 14 days in patients with zone I involvement is an indication for radical surgery (Figure 7)2,4-6,14,23.
In this study, the surgical results evaluated using the Whitaker classification15 followed the trend of recently published results5; in both studies, the outcomes of treatment (conservative and radical) were classified on average as Whitaker category I (no need for surgical revision). However, as the Whitaker classification system evaluates the surgical result at a specific time (static evaluation) and craniofacial fibrous dysplasia characteristically displays progressive growth (dynamic process), the results reported by our group and others5 may change as the duration of postoperative follow-up increases. Therefore, even if classified into category I, a patient should be monitored regularly for a long period because of the risks of new bone lesions, recurrence, and malignant progression1,2,8,16,17.
CONCLUSIONS
In this retrospective study, we presented an algorithm for the treatment of craniofacial fibrous dysplasia based on 16 years of experience. According to the surgical results presented and discussed in this study, the surgical approach for these patients should be individualized in line with the predetermined criteria of preserving function and facial harmony and aesthetics.
REFERENCES
1. Hunt JA, Hobar PC. Common craniofacial anomalies: conditions of craniofacial atrophy/hypoplasia and. Plast Reconstr Surg. 2003;111(4):1497-508.
2. Ricalde P, Magliocca KR, Lee JS. Craniofacial fibrous dysplasia. Oral Maxillofac Surg Clin North Am. 2012;24(3):427-41.
3. Béquignon E, Cardinne C, Lachiver X, Wagner I, Chabolle F, Baujat B. Craniofacial fibrous dysplasia surgery: A functional approach. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;2013 Jul 18. [Epub ahead of print] 4. Fattah A, Khechoyan D, Phillips JH, Forrest CR. Paediatric craniofacial fibrous dysplasia: the Hospital for Sick Children experience and treatment philosophy. J Plast Reconstr Aesthet Surg. 2013;66(10):1346-55.
5. Gabbay JS, Yuan JT, Andrews BT, Kawamoto HK, Bradley JP. Fibrous dysplasia of the zygomaticomaxillary region: outcomes of surgical intervention. Plast Reconstr Surg. 2013;131(6):1329-38.
6. Valentini V, Cassoni A, Marianetti TM, Terenzi V, Fadda MT, Iannetti G. Craniomaxillofacial fibrous dysplasia: conservative treatment or radical surgery? A retrospective study on 68 patients. Plast Reconstr Surg. 2009;123(2):653-60.
7. Zeng HF, Lu JJ, Teng L, Jin XL, Xu JJ, Zhang C, et al. Surgical treatment of craniomaxillofacial fibrous dysplasia: functionally or aesthetically? J Craniofac Surg. 2013;24(3):758-62.
8. Kruse A, Pieles U, Riener MO, Zunker Ch, Bredell MG, Grätz KW. Craniomaxillofacial fibrous dysplasia: a 10-year database 1996-2006. Br J Oral Maxillofac Surg. 2009;47(4):302-5.
9. Raposo-Amaral CE, de Campos Guidi M, Warren SM, Almeida AB, Amstalden EM, Tiziane V, et al. Two-stage surgical treatment of severe cherubism. Ann Plast Surg. 2007;58(6):645-51.
10. Voegels RL, Thomé DC, Imamura R, Nakano EA, Butugan O, Miniti A. Displasia fibrosa da região da cabeça e pescoço: aspectos clínico e cirúrgico. Rev Bras Otorrinolaringol. 1998;64(4 Pt 1):362-5.
11. Antunes AA, Romualdo Filho J, Antunes AP. Displasia fibrosa óssea: estudo retrospectivo- revisão de literatura. Rev Bras Cir Cab Pesc. 2004;33(1):21-6.
12. Botelho RA, Tornin OS, Yamashiro I, Menezes MC, Furlan S, Ridelenski M, et al. Características tomográficas da displasia fibrosa craniofacial: estudo retrospectivo de 14 casos. Radiol Bras. 2006;39(4):269-72.
13. Prado Ribeiro AC, Carlos R, Speight PM, Hunter KD, Santos-Silva AR, de Almeida OP, et al. Peritrabecular clefting in fibrous dysplasia of the jaws: an important histopathologic feature for differentiating fibrous dysplasia from central ossifying fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(4):503-8.
14. Chen YR, Noordhoff MS. Treatment of craniomaxillofacial fibrous dysplasia: how early and how extensive? Plast Reconstr Surg. 1990;86(5):835-42.
15. Whitaker LA, Bartlett SP, Schut L, Bruce D. Craniosynostosis: an analysis of the timing, treatment, and complications in 164 consecutive patients. Plast Reconstr Surg. 1987;80(2):195-212.
16. Cheng J, Wang Y, Yu H, Wang D, Ye J, Jiang H, et al. An epidemiological and clinical analysis of craniomaxillofacial fibrous dysplasia in a Chinese population. Orphanet J Rare Dis. 2012;7:80.
17. Ma J, Liang L, Gu B, Zhang H, Wen W, Liu H. A retrospective study on craniofacial fibrous dysplasia: preoperative serum alkaline phosphatase as a prognostic marker? J Craniomaxillofac Surg. 2013;41(7):644-7.
18. Wei YT, Jiang S, Cen Y. Fibrous dysplasia of skull. J Craniofac Surg. 2010;21(2):538-42.
19. Lee JS, FitzGibbon E, Butman JA, Dufresne CR, Kushner H, Wientroub S, et al. Normal vision despite narrowing of the optic canal in fibrous dysplasia. N Engl J Med. 2002;347(21):1670-6.
20. Amit M, Collins MT, FitzGibbon EJ, Butman JA, Fliss DM, Gil Z. Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia--a meta-analysis. PLoS One. 2011;6(9):e25179.
21. Chen YR, Breidahl A, Chang CN. Optic nerve decompression in fibrous dysplasia: indications, efficacy, and safety. Plast Reconstr Surg. 1997;99(1):22-30.
22. Tan YC, Yu CC, Chang CN, Ma L, Chen YR. Optic nerve compression in craniofacial fibrous dysplasia: the role and indications for decompression. Plast Reconstr Surg. 2007;120(7):1957-62.
23. Choi JW, Lee SW, Koh KS. Correction of proptosis and zygomaticomaxillary asymmetry using orbital wall decompression and zygoma reduction in craniofacial fibrous dysplasia. J Craniofac Surg. 2009;20(2):326-30.
24. Sarnat BG, Bradley JP. The upper face and orbit: clinical considerations. In: Sarnat BG, Bradley JP, eds. Craniofacial Biology and Craniofacial Surgery. London: World Scientific; 2010. p.335-53.
1. MD. Aspiring Member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), Resident Physician of Plastic Surgery, "Prof. Dr. Cassio M. Raposo do Amaral" Plastic Surgery Service, Institute of Craniofacial Plastic Surgery, SOBRAPAR Hospital, Campinas, SP, Brazil
2. MD. Aspiring Member of the .SBCP, Resident Physician of Plastic Surgery, "Prof. Dr. Cassio M. Raposo do Amaral" Plastic Surgery Service, Institute of Craniofacial Plastic Surgery, SOBRAPAR Hospital, Campinas, SP, Brazil
3. MD. Aspiring Member of the .SBCP, Resident Physician of Plastic Surgery, "Prof. Dr. Cassio M. Raposo do Amaral" Plastic Surgery Service, Institute of Craniofacial Plastic Surgery, SOBRAPAR Hospital, Campinas, SP, Brazil
4. MD, MSc. Full member of the SBCP and of the Brazilian Association for Cranio-Maxillofacial Surgery (ABCCMF), Master of Surgery from the University of Campinas (UNICAMP), Head of the "Prof. Dr. Cassio M. Raposo do Amaral" Plastic Surgery Service, Institute of Craniofacial Plastic Surgery, SOBRAPAR Hospital, Campinas, SP, Brazil
5. MD. Full member of the SBCP, Head Tutor of the residents in the "Prof. Dr. Cassio M. Raposo do Amaral" Plastic Surgery Service, Institute of Craniofacial Plastic Surgery, SOBRAPAR Hospital, Campinas, SP, Brazil
6. MD, PhD. Head of the Department of Pediatric Neurosurgery, Hospital das Clínicas, State University of Campinas (HC-UNICAMP), PhD in Medical Pathophysiology with a major in Neuroscience, UNICAMP, Neurosurgeon of the Institute of Craniofacial Plastic Surgery, SOBRAPAR Hospital, Campinas, SP, Brazil
7. MD, PhD. Full member of the SBCP and ABCCMF, PhD in Clinical Surgery, University of São Paulo (USP), Head Tutor and Vice President of the Institute for Craniofacial Plastic Surgery, SOBRAPAR Hospital, Campinas, SP, Brazil.
Correspondence to:
Cassio Eduardo Raposo-do-Amaral
Av. Adolpho Lutz, 100 - Cidade Universitária
Campinas, SP, Brazil - CEP 13083-880 - CP 6028
E-mail: cassioraposo@hotmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: 17/9/2013
Article accepted: 5/10/2013
The research was performed at the Institute for Craniofacial Plastic Surgery, SOBRAPAR Hospital, Campinas, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter