

Ideas and Innovation - Year 2013 - Volume 28 -
Breast Q questionaire ,translation process to portuguese language and their application on breast cancer patients
Tradução do Questionário Breast-Q para a língua portuguesa e sua aplicação em mulheres com câncer de mama
ABSTRACT
According to the Ministry of Health are expected in Brazil, in 2012, 52,680 new cases of breast cancer with an estimated risk of 52 cases per 100 000 women. Recently the quality of life (QOL), now considered a parameter increasingly important to evaluate the result of treatment. To assess the QOL of women with breast cancer, Pusic et al. developed a questionnaire called BREAST-Q, related to breast surgery, and divided into three modules: an augmentation, reduction and reconstruction with independent scales to assess the major problems affecting patients undergoing each type of procedure, either in the pre and postoperatively. This questionnaire was developed in English but now has versions in several languages. We present the translation process for the construction of the Portuguese version, which took part in the translation, backward translation and testing to obtain the final version and its approval by the owner (MAPI Trust© _Mapi Research Institute 2002). Translation is the beginning of the process of validation of questionnaires and their use in order to assess the impact of treatment of breast cancer from the perspective of Brazilian women.
Keywords: Quality of Life. Questionnaires. Breast Neoplasms.
RESUMO
Segundo o Ministério da Saúde, esperavam-se para o Brasil, em 2012, 52.680 casos novos de câncer de mama, com risco estimado de 52 casos a cada 100 mil mulheres. Recentemente, a qualidade de vida (QV), passou a ser considerado um parâmetro cada vez mais importante para avaliar o resultado do tratamento. Para avaliar a QV das mulheres com câncer de mama, Pusic et al. desenvolveram um questionário denominado BREAST-Q, relacionado à cirurgia de mama, e dividido em três módulos: aumento, redução e reconstrução com escalas independentes para avaliar os maiores problemas que afetam as pacientes submetidas a cada tipo de procedimento, tanto no pré como no pós-operatório. Este questionário foi desenvolvido na língua inglesa, mas já possui versões em vários idiomas. Apresentamos o processo de tradução para a construção da versão na língua portuguesa, do qual fizeram parte a tradução, tradução reversa e testagem até a obtenção da versão final e sua aprovação pelo proprietário (MAPI Trust© Mapi Research Institute 2002). A tradução é o início do processo de validação dos questionários e sua utilização com o objetivo de avaliar o impacto do tratamento do câncer de mama sob a perspectiva das mulheres brasileiras.
Palavras-chave: Qualidade de Vida. Questionários. Neoplasias da Mama.
Breast cancer is an increasingly common problem worldwide and can have a devastating impact on affected women. In Brazil, in 2012, breast cancer was estimated to be the second most frequent cancer in women, behind only nonmelanoma skin cancer1. Historically, the parameters used in evaluating the results of cancer treatment were disease-free survival and overall survival2; however, today, they are considered insufficient With the available therapeutic options and rapid advances in breast surgery procedures, quality of life (QOL) is being considered an important parameter3,4 that helps physicians and patients decide on the most appropriate approach. There are several definitions in the literature for QOL; however, those definitions are considered subjective and multidimensional, and may be strongly influenced by sociocultural factors2,3.
Currently, patient satisfaction and health-related QOL have become important tools for assessing the results and success of breast surgery, whether cosmetic or reconstructive5. Thus, treatment results are evaluated from the patient's perspective, and comparisons between different surgical techniques, studies, and populations can be made. With this in mind, Pusic et al.6 , from the Memorial Sloan-Kettering Cancer Center, together with researchers at the University of British Columbia, presented a new questionnaire called BREAST-Q. This questionnaire is related to breast surgery and divided into three modules: augmentation, reduction, and reconstruction, with separate scales to assess the major problems affecting patients undergoing each type of procedure, both pre- and postoperatively
Recently, a new module was included for mastectomy patients who did not undergo breast reconstruction7. This research tool can provide valuable information on the QOL of women treated for breast cancer, assessing their physical, psychosocial, and sexual well-being, as well their satisfaction with their breasts, results, and care throughout the treatment. Plastic surgeons can assess their patients and obtain feedback to enhance their individual practice. It can also provide essential information about the impact and effectiveness of breast surgery and the patient's perspective. Researchers and doctors worldwide can use it for the benefit of patients. QOL-related studies may provide evidence for discriminating differences in short- and long-term treatment outcomes. However, to be appropriately used in clinical practice and research in Brazil, translation of the BREAST-Q questionnaire into Portuguese is necessary, and this process will be described in this article.
OBJECTIVE
This study aims to present the translation process of the BREAST-Q questionnaire into the Portuguese language .
METHODS
The study was approved by the research ethics committee of INCA. The translation into Portuguese was performed after obtaining prior authorization, following the norms of the institution that owns the copyright (Mapi Trust©, Mapi Research Institute 2002) (Figure 1), and consisted of three phases. In phase 1, the English-to-Portuguese translation was done by three professional translators, who, after a comparison of the versions, developed a consensus version along with the authors. In phase 2, back-translation was performed by a second team of different translators and, after comparison with the originals and new consensus with the authors, produced the final version in Portuguese. Finally, in phase 3, the Portuguese version of each questionnaire was applied in five patients7.
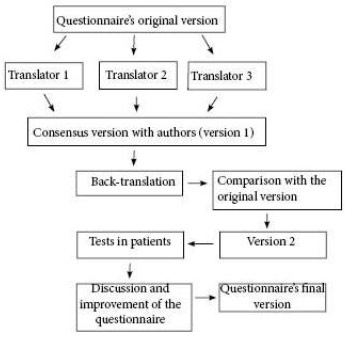
Figure 1 - Algorithm of the linguistic validation process.
After testing, adjustments to the literal translation were made with the goal of providing easily understandable synonyms, keeping the meaning of the sentences of the original version, which cannot be modified. The BREAST-Q questionnaires will be validated in breast cancer patients treated at the Mastology and Plastic Surgery Services of INCA. An ongoing prospective study will be conducted by analysis of the responses to the questionnaires appropriate for the pre- and postoperative phases of mastectomy and immediate and delayed breast reconstruction. Data analysis will be performed following the instruction guide and a program provided by the owner of the questionnaire (Mapi Trust), which can convert the responses into numerical scores that can be interpreted. The statistical analysis of the linear variables will be stratified into ranges.
RESULTS
When the final Portuguese versions of the questionnaires were finalized, they were sent for approval to the Mapi Trust and Dr. Andrea Pusic, who owns the copyright. The questionnaires have been approved, and their franchised use is now allowed for researchers who want to use this Portuguese version for women undergoing mastectomy, breast reconstruction, breast augmentation, and breast reduction, subject to authorization by the copyright holders .
DISCUSSION
Many women with breast cancer can be treated with curative intent, which makes aspects of QOL, especially in the long-term, very important3. Considerable physical and psychological repercussions are associated with the treatment of this condition, contributing to a negative perception in their QOL2,4-7. The use of validated questionnaires is well established in the literature as an appropriate method for evaluating the QOL. Thus, tools with established reliability that have been validated and translated into Portuguese are required for introducing specific reviews for breast surgery in the country .
Published questionnaires that evaluate the QOL can be divided into generic and specific. The generic type assesses global aspects related to various domains such as physical, social, psychological, emotional, and sexual. We can highlight The Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36)4,8 and the World Health Organization Quality of Life (WHOQOL) questionnaires. Specific questionnaires assess other aspects of the QOL. Those related specifically to women with breast cancer include the European Organization for Research and Treatment of Breast Cancer-Specific Quality of Life Questionnaire (EORTC QLQ-BR23)4,9 (EORTC Quality of Life Group, 1996) and the Functional Assessment of Cancer Therapy-Breast (FACT-B)4,9.
Studies using the BREAST-Q6,7 questionnaire can provide plastic surgery professionals a better understanding of various surgical techniques, types of complementary treatments, comparison of outcomes and treatment-related studies of breast cancer surgery, and the QOL of affected patients. They can also assess the impact and effectiveness of the treatment in the patient's perspective, an important aspect in previous studies10. The scores on the questionnaires may vary from 0 to 100. A higher score means a higher satisfaction, or a better QOL. The clinical significance of the score obtained and the lowest clinically significant difference between scores is not yet defined; however, a study that will assist in better understanding these data is ongoing. Nevertheless, the interpretation of the clinical significance of this score is facilitated by a recent study of 2000 patients at the Memorial Sloan-Kettering Cancer Center. This study suggests that differences of 5-10 points, in multi-item scales, can be considered a small change, 10-20 as moderate, and >20 points as a major change in QOL.
Some authors suggest that a more active participation of the patient throughout treatment periods, such as guiding them on their surgical options or type of complementary treatments, leads to better results. It may also be useful for the personal improvement of each professional in this area, with the main objective of providing the best treatment for patients in each specific situation.
CONCLUSION
Use of the BREAST-Q questionnaire by Brazilian plastic surgeons can provide important information on the treatment of patients with breast cancer and their QOL. The pre- and postoperative periods of mastectomy and breast reconstruction, and also the different reconstruction techniques, can be compared, and their impact on the lives of women with breast cancer can be better understood through their own point of view.
REFERENCES
1) Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2012: incidência de câncer no Brasil. Rio de Janeiro: Inca; 2011. 118p.
2) Conde DM, Pinto-Neto AM, Júnior RF, Aldrighi JM. Qualidade de vida de mulheres com câncer de mama. Rev Bras Ginecol Obstet. 2006;28(3):195-204.
3. Rocha LR, Veiga DF, e Oliveira PR, Song EH, Ferreira LM. Health Service Quality Scale: Brazilian Portuguese translation, reliability and validity. BMC Health Serv Res. 2013;13:24.
4. Majewski JM, Lopes ADF, Davoglio T, Leite JCC. Qualidade de vida em mulheres submetidas à mastectomia comparada com aquelas que se submeteram à cirurgia conservadora: uma revisão de literatura. Ciênc Saúde Coletiva. 2012;17(3):707-16.
5. Pusic AL, Chen CM, Cano S, Klassen A, McCarthy C, Collins ED, et al. Measuring quality of life in cosmetic and reconstructive breast surgery: a systematic review of patient-reported outcomes instruments. Plast Reconstr Surg. 2007;120(4):823-37.
6. Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345-53.
7. Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL. The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg. 2012;129(2):293-302.
8. Veiga DF, Veiga-Filho J, Ribeiro LM, Archangelo I Jr, Balbino PF, Caetano LV, et al. Quality-of-life and self-esteem outcomes after oncoplastic breast-conserving surgery. Plast Reconstr Surg. 2010;125(3):811-7.
9. Macadam SA, Ho AL, Cook EF Jr, Lennox PA, Pusic AL. Patient satisfaction and health-related quality of life following breast reconstruction: patient-reported outcomes among sa
1. MSc, Plastic Surgeon
2. General Surgeon and Physician; Resident, Reconstructive Plastic Surgery and Microsurgery Service, INCA
3. MSc, Medical Biosciences, UNICAMP; Head, Technical-Scientific Division, Cancer Hospital IV, INCA/MS, Coordinator of the Research Ethics Committee, INCA/MS
4. Plastic Surgeon, Full Member of the SBCP; Head of the Reconstructive Plastic Surgery and Microsurgery Service, INCA
5. Lecturer in Gynecology, UNICAMP; Full Professor of Gynecology, Faculty of Medical Sciences, UNICAMP
Juliano Carlos Sbalchiero
Praça Cruz Vermelha, 23 - Centro
CEP 20230-130 Rio de Janeiro, RJ, Brasil
Article received: 9/10/2013
Article accepted 23/11/2013


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter