

Original Article - Year 2014 - Volume 29 -
Smoking influence on the skin quality of white women
Influência do tabagismo na qualidade da pele de mulheres brancas
ABSTRACT
INTRODUCTION: This study aimed to evaluate the influence of smoking on the skin quality of Caucasian women by analyzing collagen, elastic fibers, and vascularization.
METHODS: Histological and morphometric analysis was carried out on the pre-auricular flaps of 78 women undergoing facial cosmetic surgery. The patients were paired (n = 39) based on age, to form a group of smokers and another of non-smokers. Collagen types I and III were quantified using Picrosirius Ultra red staining, Weigert staining was used to analyze elastic material, and anti-CD34 immunostaining highlighted blood vessels.
RESULTS: Dermal density of both types of collagen decreased with age in both groups. The decrease in type I was higher in the dermis of smokers aged 50-59 years (p = 0.019). Elastic material increased in the dermis with age, becoming fragmented, thickened, and disorganized, particularly in 50-59-year-old smokers (p = 0.006). Moreover, in smokers, a gradual and significant increase in the number of microcirculatory vessels also observed (p = 0.008).
CONCLUSION: The skin of women who smoke contains less collagen (type I in particular), more degenerated, fragmented and thickened elastic material, and a greater number of blood vessels.
Keywords: Skin; Women; Collagen; Elastic tissue; Microcirculation; Smoking.
RESUMO
INTRODUÇÃO: Avaliar a influência do tabagismo na qualidade da pele de mulheres brancas analisando o colágeno, as fibras elásticas e a vascularização.
MÉTODOS: Realizou-se análise histológica e morfométrica de 78 retalhos pré-auriculares de mulheres brancas que se submeteram à cirurgia estética facial. As pacientes foram pareadas conforme a idade, constituindo dois grupos (n=39), um de fumantes e um de não fumantes. Utilizaram-se a coloração de Picrosírius Ultra red para quantificar os colágenos I e III, a coloração de Weigert para a análise do material elástico e o imunomarcador anti CD34 para evidenciar os vasos sanguíneos.
RESULTADOS: Foi possível verificar que em fumantes e não fumantes há diminuição da densidade de colágeno dérmico com o envelhecimento, tanto na fração I quanto na III. A diminuição da fração I é maior na derme de fumantes, na faixa etária de 50 a 59 anos (p=0,019). O material elástico aumenta na derme com o passar dos anos, tornando-se fragmentado, espessado e desorganizado, de modo mais acentuado na derme das fumantes, na mesma faixa etária (p=0,006) e, finalmente, existe um aumento gradativo do número de vasos na microcirculação, mais significativo nas fumantes (p=0,008).
CONCLUSÃO: A pele de mulheres fumantes contém menos colágeno (especialmente do tipo I), mais material elástico degenerado, fragmentado e espessado e maior número de vasos sanguineos.
Palavras-chave: Pele; Mulheres; Colágeno; Tecido elástico; Microcirculação; Tabagismo.
The complex process of healing, defined as "the closing of tissue failures or replacing the tissue destroyed with fibrous tissue or another that is equal, thus reassembling the injured portions," is essential for survival following trauma with tissue injury1.
Smoking, along with several other factors, can negatively influence this process2.
The synthesis of subcutaneous collagen is significantly inhibited in smokers3. Cadmium, a component of tobacco, has been associated with the inhibition of pro-collagen production by fibroblasts4.
The association between smoking and healing impairment is well known in clinical practice, and is a reason for concern among plastic surgeons. Smoking has been related to higher risk of complications associated with healing such as skin necrosis, infection of the surgical site, dehiscence walls, thick scars, and delayed epidermal healing5-8. Experimentally, it was found that nicotine administration in rats increases the area of necrosis in skin flaps9-11.
Maintenance of the endothelium and microcirculation integrity are required for nutrient transport and supply, as well as the removal of metabolites from tissues. It has been clinically shown that collagen deposition is directly and significantly correlated to oxygen tension and perfusion measures12.
Nicotine has vasoconstrictor activity, reduces pO2 in tissues, increases platelet aggregation, and diminishes the proliferation of macrophages and fibroblasts13. Fibroblasts require a pO2 of 15mmHg for cell division and 15-30mmHg for collagen synthesis. This suggests that the vasoconstriction caused by nicotine may contribute to decreased oxygen tension and impair wound healing13.
Exposure of dermal fibroblasts to tobacco has been linked with decreased synthesis of type I and III collagen2,14-19. Sorensen et al. observed in smokers a decrease in inflammation, contraction, fibroblast proliferation, and vitamin C18-19. In our line of research, studies carried out with rats receiving nicotine showed that collagen density was not affected, but did result in a decreased fibroblast population20-21.
The catabolism of collagen is carried out by a series of proteolytic enzymes belonging to the metallo-proteinase (MMP) group, which are produced by fibroblasts and other cell types. Smoking has already been identified as interfering with MMP-1, MMP-2, MMP-3, MMP-7, and MMP-814,15,22. Besides affecting the extracellular matrix, smoking has also been found to alter endothelial and fibroblastic function23.
Nicotine's vasoconstriction effect has been questioned. According to Usuki et al., small doses of nicotine would result in increased skin blood flow24, while Sørensen et al. reported that the administration of nicotine does increase blood flow, but that smoking decreases it by reducing tissue pO218.
Chronic exposure to nicotine decreases the regulation of cholinergic nicotinic receptors in the endothelium and induces a reduction in the serum levels of vascular endothelial growth factor (VEGF), thus impairing angiogenesis. Acute exposure, in turn, seems to promote increased capillary density25. Therefore, low concentrations of nicotine could accelerate angiogenesis and, in this condition, improve remodeling and healing26.
The relationship between skin aging and smoking was first described in 1856 by Solly27. In 1985 Model established criteria to define the "smoker face": salient wrinkles, sunken eyes with bony prominences, and atrophic/ grayish skin or plethoric skin28.
There are two processes regulating skin aging: an intrinsic process, which consists of a slow degenerative mechanism present in all tissues and organs, and an extrinsic process, which is caused by exposure to a variety of agents, such as photo-exposure and smoking29. The intensity of ageing is related to both the number of cigarettes/day and to the smoking duration30-32.
In the skin, the first clinical signs of aging are dryness, loss of firmness and elasticity, and the appearance of wrinkles, especially in photo-exposed areas. These are accompanied by histological changes that vary depending on the part of the skin being analyzed and the age of the individual 33. Senile skin shows flattening of the dermal-epidermal junction, marked atrophy, and loss of elasticity in dermal connective tissues, associated with reduction and disorganization of extracellular matrix components, type I and III collagens, elastic fibers, proteoglycans, and glycosaminoglycans16,33. Raduan et al., considered tobacco as an independent factor involved in the appearance of skin wrinkles34.
For a long time, aging has been related to oxidative stress, which is significantly accelerated by the smoking-related increase in free radical formation35,36. These processes can be further enhanced by external factors, such as UV light35.
Baroni et al., reported that aging causes a reduction in the density, fragmentation, and a disorganization of dermal collagen. The authors observed an increased density of elastic material, but its disorganization led these authors to call it elastotic material and not elastic fibers37.
Just et al., consider smoking as an independent factor for the increase of elastic fibers in the reticular dermis of skin that is not photo-exposed and as an additive factor for the aging of photo-exposed skin38.
When searching for information about the influence of smoking on aging, we found major methodological problems, including small and inadequate samples, and the lack of control for interference factors.
OBJETIVE
The aim of this study is to compare the skin quality of smoking vs. nonsmoking Caucasian women.
METHOD
The study analyzed slides prepared for the investigation registered under the protocol number 0541.0.084.000-09 in CONEP and number 5430 in the Human Research Ethics Committee of the Pontifical Catholic University of Paraná, after approval under the protocol number 0003880/10.
All patients, a total of 250, were included in the study after having signed the informed consent form to authorize the use of pre-auricular skin flaps removed during facial plastic surgery. A questionnaire completed by the patients allowed us to identify smokers and non-smokers. Interference factors, such as hormone replacement therapy, use of corticosteroids or other medications, previous facial aesthetic treatment, and intense sun exposure were used as exclusion criteria.
The slides containing histological sections were stained with hematoxylin-eosin (HE) and Picrosirius-red. HE staining allowed for the recognition of general skin structures, and under polarized light Picrosirius-red was used to identify the density of type I and III collagen (the main constituents of this tissue). Weigert's hematoxilyn was used to identify elastic material, and blood vessels in the sections were identified through immunostaining with anti-CD34 antibody. Five fields of each section were analyzed, and the average density of collagen and elastic material was obtained by calculating the percentage of the area examined, and the average number of vessels was determined by counting the average vessels in the fields examined. Evaluation was performed using a blind experimental design, with the investigator being unaware of which samples belonged to which group. The Image Plus® 4.5 program for Windows® from Media Cybernetics was used for the analysis.
The data were tabulated and statistically analyzed.
The results are expressed as averages, medians, minimum and maximum values, and standard deviations. To compare groups defined by their age range, the Kruskal-Wallis non-parametric test was used. The non-parametric Mann-Whitney test was used to compare groups of smokers and non-smokers within each age range. For parametric results, we used the Student's t test. Values of p <0.05 indicated statistical significance. The data were analyzed by the computational program Statistics v.8.0.
RESULTS
When considering the inclusion criteria, we were able to pair 78 women out of our original sample of 250; 39 non-smokers and 39 smokers.
Allocated by age, we were able to study three subgroups of smokers and non-smokers. Nine pairs were observed in the 40-49 age group, 24 in the 50-59 age group, and six in the 60-69 age group. We were not able to study patients over the age of 70, as the sample size was insufficient to carry out analysis when excluding confounding factors.
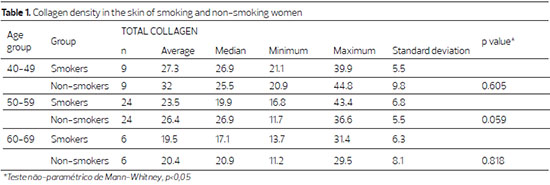
The collagen density in the dermis decreased with advancing age in both groups, though not significantly in the non-smoking group (p = 0.211), and significantly in the smoking group (p = 0.042). When comparing smokers and non-smokers within the same age group, the difference was not significant (Table 1).
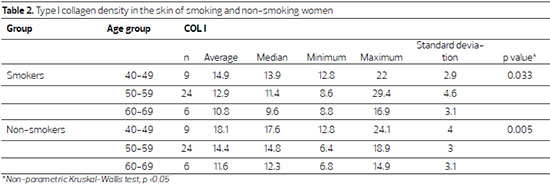
A significant reduction in type I collagen was observed in the skin of non-smokers with advancing age (p = 0.033), an effect more pronounced in the skin of smoking women (p = 0.005)(Table 2).
Although type I collagen density was lower in smokers, the within-age group comparison showed that collagen type I density was lower in the skin of smokers aged 40-49 (p = 0.077), and was significantly lower in smokers between 50 and 59 years of age (p = 0.019) (Table 3; Figure 1).
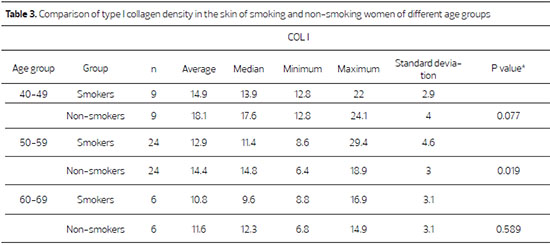
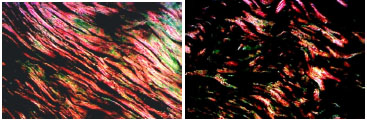
Figure 1. Photomicrographs of histological sections stained with Picrosirius-red, showing the skin section of a smoker (upper panel) and of a non-smoker (lower panel), aged between 50 and 59 years old (red = type I collagen and green = type III collagen)(200X).
The analysis of the elastic material showed no significant difference in the skin of patients aged between 40 and 49 years (p = 0.071), though there is a significant increase in the skin of women aged 50-59 (p = 0.006), which is maintained with increasing age (Table 5). Within-group analysis showed that the gain of elastic material in smoking patients aged 50-59 was not significant compared to that of their 40-49 year old counterparts (p = 0.401). However, this gain was significant when compared to the skin of patients aged between 60 and 69 years (p = 0.032). The same tendency was observed in the skin of non-smoking patients, although the overall percentage of elastic material in these women was lower; the skin of patients aged 40-49 did not have significantly different levels of elastic material compared to patients aged 50-59 (p=0.494). The comparison of women aged 50-59 with those aged 60-69 found a significant difference between the two groups (p = 0.044)(Figure 2).
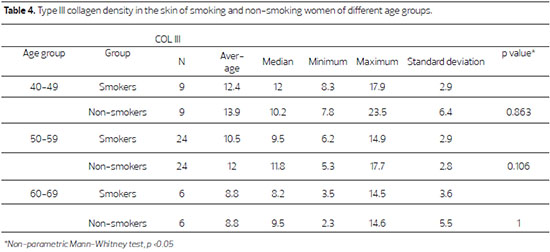
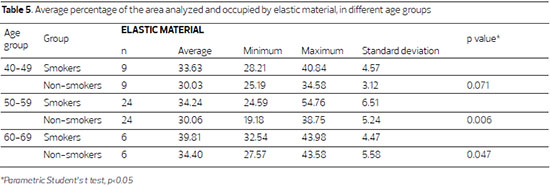
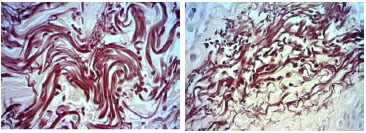
Figure 2. Fotomicrografias de cortes histológicos de pele tratados pela hematoxilina de Weigert, a imagem superior, de paciente não fumante e a inferior de paciente fumante, da faixa 50 a 59 anos. (400X)
Although type III collagen density diminishes with advancing age, this change was not significant in the skin of smokers and non-smokers (p = 0.124, and p = 0.526, respectively). The comparison between different age groups was also not significant (Table 4).
In smokers, the average number of vessels per field is higher in all of the evaluated age groups compared to non-smokers, and this increase is significant in the 50-59 age group (p = 0.008)(Table 6; Figure 3).
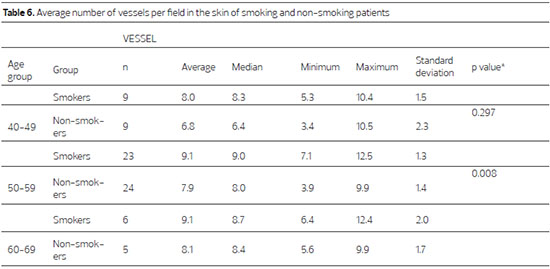
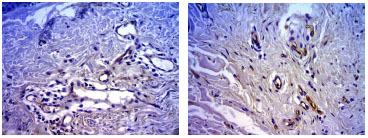
Figure 3. Photomicrograph of skin sections of a non-smoker (upper panel) and a smoker (lower panel) in the 50-59 age group, stained with anti-CD 34 antibody (400X).
DISCUSSION
Analyzing the results obtained in their studies, Baroni et al., concluded that skin aging induces qualitative and degenerative changes of the dermis, reduces type I and III collagen, as well as total collagen, and causes a disorganization and fragmentation of collagen fibers, especially from the age of 60 years37. Smoking impairs collagen synthesis by reducing the number of fibroblasts2,3,14-21.
Broniarczyk-Dyla et al., stated that aging is accelerated in post-menopausal women due to a reduction in collagen and ovarian estrogen production39.
It is expected that in addition to the physiological loss of collagen caused by aging, women smokers will show more pronounced loss. In this study, we observed that the skin of smokers had lower collagen levels, although the difference was not significant. However, collagen type I, which is structurally more organized, was lower in the skins of smokers aged 50-59 (p = 0.019). While this could not be confirmed in the other age groups, there was a tendency to have less collagen among smokers, though the sample was quite small. Although several authors have reported that dermal fibroblast exposure to tobacco decreases the synthesis of type I and type IIII collagen2,14-19, this study found a reduction in both smokers and non-smokers, although this decrease was more pronounced in smokers.
The physiological elasticity and resilience of the dermis are due to elastic fibers40. Degeneration of elastic fibers, decrease in collagen, particularly type I collagen, and disorganization reduces the elasticity of the skin and enhances the formation of wrinkles41.
Elastin network density increases from birth until it reaches its peak volume at around age 20 for women and age 40 for men42. With increasing age, the elastic fibers become thickened, tangled, degraded, and dysfunctional43. Biochemically, the elastotic material resembles elastin, although disorganized and abnormal in the ratio of various constituents43. In areas with actinic degeneration, the elastic fibers become thicker, with a progressive increase in the mass of abnormal granular elastotic material41.
Smoking is considered an independent factor for the increase of elastic fibers in the reticular dermis of not photo-exposed skin, and as an additive factor for the aging of photo-exposed skin38.
Ortolan's study reported that aging caused an increase in elastic material density, with quality modifications in the organization of elastic fibers44. Baroni et al., observed an increased density of elastic material, which was, however, disorganized, leading the authors to term it elastotic materials and non-elastic fibers37.
In this sample, the skin dermis of smoking and non-smoking women showed an increase in elastic material density with age, which was particularly pronounced in the smokers. A very significant difference was detected in smokers belonging to the 50-59 age group (p = 0.006).
Longtime smokers frequently showed some degree of chronic obstructive pulmonary disease (COPD). Maclay et al. found increased elastin degradation in the skin of COPD-patients when compared to controls45. This report reinforces the previous findings regarding the disorganization and degradation of elastic fibers. Although Rosado et al. studied young adult male smokers (aged between 23 and 36 years old), they observed that elastic material increased 42.5% when compared to non-smoking controls46.
Several authors have reported that aging promotes a decrease in cutaneous microcirculation with reduced capillary loops, vascular dilatation, and endothelial proliferation with decreased functional reserve47-49. Studies in our line of research showed no significant changes in the blood vessel density of aging dermis44. In this study, we observed that the skin dermis of smokers contained a higher number of vessels, and this was very significant in the 50-59 age group (p=0.008). It is possible that a certain degree of tissue hypoxia stimulates neoangiogenesis.
Collagen synthesis and organization is dependent on oxygen12, and so smoking could be considered to accelerate aging by interfering with collagen organization if nicotine promotes oxidative stress and vasoconstriction. Interfering factors greatly impair the study of this link in clinical practice. For example, most older patients present with age-related diseases and many use medication, both of which can interfere with collagen and vascularization. Moreover, many have already undergone one or even several rejuvenation treatments. Once exclusion criteria are applied, it becomes clear that very large samples are needed to ensure a sufficient number of patients for analysis. In this study, 250 women initially met the requirements for surgical treatment; however, once the exclusion criteria were applied, 68.8% of the sample was lost, and only 78 women remained. Caucasian women were primarily sampled due to their high density in the population of our region, and it is very likely that this behavior is different in women belonging to other racial groups. Girardeau et al., showed that there are differences in superficial epidermis, dermal functions, and cellular interactions between African and Caucasian skin50.
In this cohort, we verified that aging decreases dermal collagen density, both types I and III, in smokers and non-smokers. However, this decrease is more pronounced in the dermis of smoking patients. Elastic material increases in the dermis over the years, becoming fragmented, thickened and disorganized, and this is more accentuated in the dermis of smokers, along with a gradual but significant increase in the number of microcirculatory vessels.
CONCLUSION
The skin of women who smoke contains less collagen, particularly type I, and contains more elastic material that is degenerated, fragmented, and thickened, as well as a greater number of blood vessels.
REFERENCES
1. Singer AJ, Clark RAF. Mechanism of disease: cutaneous wound healing. N Engl J Med. 1999;340(10):738-46.
2. Tipton DA, Dabbous MK. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblast in vitro. J Periodontol. 1995;66(12):1056-64.
3. Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery. 1998;123(4):450-5.
4. Chambers RC, McAnulty Rj, Shock A, Campa JS, Newman Taylor AJ, Laurant GJ. Cadmium selectively inhibits fibroblast procollagen production and proliferation. Am J Respir Cell Mol Biol. 1998;19(3):498-506.
5. Selber JC, Kurichi JE, Vega SJ, Sonnad SS, Serletti JM. Risk factors and complications in free TRAM flap breast reconstruction.Ann Plast Surg. 2006;56(5):492-7.
6. Spear SL, Ducic I, Cuoco F, Hannan C. The effect of smoking on flap and donor-site complications in pedicled TRAM breast reconstruction. Plast Reconstr Surg. 2005;116(7):1873-80.
7. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled Trial. Ann Surg. 2003;238(1):1-5.
8. Sørensen LT, Hemmingsen U, Kallehave F, Wille-Jørgensen P, Kjaergaard J, Møller LN, et al. Risk factors for tissue and wound complications in gastrointestinal surgery. Ann Surg. 2005;241(4):654-8.
9. Campos H, Ferreira LM, Conrado dos Santos WL, Araújo MCM. Efeitos da nicotina nos retalhos cutâneos em ratos. Acta Cir Bras. 2001;16(4):206-10.
10. Eroglu L; Orak I; Turhan Haktanir N. Effect of short-term use of oral smokeless tobacco on random- pattern skin flap s. urvival in rats. Scand J Plast Rreconstr Surg Hand Surg. 2005;39(5):272-6.
11. Fan GB; Wu PL; Wang XM. Changes of oxygen content in facial skin before and after cigarette smoking. Skin Res Technol. 2012;18(4):511-5.
12. Jonsson K, Jensen JA, Goodson WH, Scheuenstuhl H, West J, Hopf HW, et al. Tissue oxygenation, anemia, and perfusion in relation to the wound healing in surgical patients. Ann Surg. 1991;214(5):605-13.
13. Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126(9):1131-4.
14. Yin L, Morita A, Tsuji T. Alterations of extracellular matrix induced by tobacco smoke extract. Arch Dermatol Res 2000;292(4):188-94.
15. Knuutinen A, Kallioinen M, Vähäkangas K, Oikarinen A. Smoking and skin: a study of the physical qualities and histology of skin in smokers and non-smokers. Acta Derm Venereol. 2002;82(1):36-40.
16. Morita A. Tobacco smoke causes premature skin aging. J Dermatol Sci. 2007;48(3):169-75.
17. Tanaka H, Ono Y, Nakata S, Shintani Y, Sakakibara N, Morita A. Tobbaco smoke extract induces premature skin aging in mouse. J Dermatol Sci. 2007;46(1):69-71.
18. Sørensen LT, Jørgensen S, Petersen LJ, Hemmingsen U, Büllow J, Loft S, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res. 2009;152:224-30.
19. Sørensen LT, Toft BG, Rygaard J, Ladelund S, Paddon M, James T, et al. Effect of smoking, smoking cessation, and nicotine patch on wound dimension, vitamin C, and systemic markers of collagen metabolism. Surgery. 2010;148(5):982-90.
20. Biondo-Simões MLP et al. A influência da nicotina na densidade de colágeno em cicatrizes cutâneas, em ratos.Rev Col Bras. 2009;36(5):425-30.
21. Biondo-Simões MLP et al. The influence of nicotine on the population of fibroblasts in cutaneous scars in rats.Acta Cir Bras. 2009;24(6):466-70.
22. Ortega YV, Tomey AV. Metaloproteinasas de la matriz y envejecimiento cutâneo. Rev Habanera Cienci. Med. 2003; 2(5). Internet. Disponível: http://www.ucmb.sld.cu/rhab/index.html. Acesso em 2011.
23. Su Y, Cão W, Han Z, Block ER. Cigarette smoke extract inhibits angiogenesis of pulmonary artery endothelial cells: the role of calpain. Am J Physiol ung Cell Mol Physiol. 2004;287(4):L794-800.
24. Usuki K, Kanekura T, Aradono K, Kanzaki T. Effects of nicotine on peripheral cutaneous blood flow and skin temperature. J Dermatol Sci. 1998;16(3):173-81.
25. Konishi H, Wu J, Cooke JP. Chronic exposure to nicotine impairs cholinergic angiogenesis. Vasc Med. 2010;15(1):47-54.
26. Morimoto N, Takemoto S, Kawazoe T, Suzuki S. Nicotine at a low concentration promotes wound healing. J Surg Res. 2008;145(2):199-204.
27. Gill JF; Yu SS; Neuhaus IM. Tobacco smoking and dermatologic surgery. J Am Acad Dermatol. 2013;68(1):167-72.
28. Model D. Smoker's face: an underrated clinical sign? Br Med J (Clin Res Ed). 1985;291(6511):1760-2.
29. Uitto J. Understanding premature skin aging. N Engl J Med. 1997;337(20):1463-5.
30. Doshi DN, Hanneman KK, Cooper KD. Smoking and skin aging in identical twins. Arch Dermatol. 2007;143:1543-6.
31. Just M, Ribera M, Monsó E, Lorenzo JC, Ferrándiz C. Effect of smoking on skin elastic fibres: morphometric and immunohistochemical analysis. Br J Dermatol. 2007;156(1):85-91.
32. Helfrich YR; Yu L; Ofori A; Hamilton TA; Lambert J; King A; Voorhees JJ; Kang S. Effect of smoking on aging of photoprotected skin: evidence gathered using a new photonumeric scale. Arch Dermatol. 2007;143(3):397-402.
33. Oriá RB, Brito GAC, Ferreira FVA, Santana EM, Fernandes MR. Estudo das alterações relacionadas com a idade na pele humana utilizando métodos de histomorfometria e auto fluorescência. An Bras Dermatol. 2003;78(4):425-34.
34. Raduan AP; Luiz RR; Manela-Azulay M. Association between smoking and cutaneous ageing in a Brazilian population. J Eur Acad Dermatol Venereol. 2008;22(11):1312-8.
35. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ . Mechanisms of photoaging and chronological skin aging.. Arch Dermatol. 2002;138(11):1462-70.
36. Nicita-Mauro V; Lo Balbo C; Mento A; Nicita-Mauro C; Maltese G; Basile G. Smoking, aging and the centenarians. Exp Gerontol. 2008;143(2):95-101.
37. Baroni ER, Biondo-Simões, ML, Auersvald A, Auersvald LA, Montemor Netto MR, Ortolan MC, et al. Influence of aging on the quality of the skin of white women: the role of collagen. Acta Cir Bras. 2012;27(10):736-40.
38. Just M; Ribera M; Monsó E; Lorenzo JC; Ferrándiz C. Effect of smoking on skin elastic fibres: morphometric and immunohistochemical analysis. Br J Dermatol. 2007;156(1):85-91.
39. Broniarczyk-Dyla G, Joss-Wichman E. Ageing of skin during menopause. J Eur Acad Dermatol Venereol. 2001;15(5):494-5.
40. Mahoney MG, Brennan D, Starcher B, Faryniarz J, Ramirez J, Parr L, et al. Extracellular matrix in cutaneous ageing: the effects of 0.1% copper-zinc malonate-containing cream on elastin biosynthesis. Exp. Dermatol. 2009;18(3):205-11.
41. Miyasaka M, Sakai S, Kusaka A, Endo Y, Kobayashi M, Kobayashi K, et al. Ultrasonic tissue characterization of photodamaged skin by scanning acoustic microscopy. Tokai J Exp Clin Med. 2005;30(4):217-25.
42. Pasquali-Ronchetti I, Baccarani-Contri M. Elastic fiber during development and aging. Microsc Res Tech. 1997;38(4):428-35.
43. Wulf HC, Sandby-Moller J, Kobayasi T, Gniadecki R. Skin aging and natural photoprotection. Micron. 2004;35(3):185-91.
44. Ortolan MCAB. Influência da idade na percentagem de material elástico e na vascularização da pele de mulheres brancas. 2012. Dissertação (Mestrado em Cirurgia) - Pontifícia Universidade Católica do Paraná.
45. Maclay JD, McAllister DA, Rabinovich R, Haq I, Maxwell S, Hartland S, et al. Systemic elastin degradation in chronic obstructive pulmonary disease. Thorax. 2012;67(7):606-12.
46. Rosado JP, Favorito LA, Cavalcanti AG, Costa WS, Cardoso LE. Structural alterations of foreskin caused by chronic smoking may explain high levels of urethral reconstruction failure using foreskin flaps. Int Braz J Urol. 2012;38(4):529-35.
47. Chung JH, Yano K, Lee MK, Youn CS, Seo JY, Kim KH, et al. Differential effects of photoaging vs intrinsic aging on de vascularization of human skin. Arch Dermatol. 2002;138(11):1437-42.
48. Chung JH, Eun HC. Angiogenesis in skin aging and photoaging. J Dermatol. 2007;34(3):593-600.
49. Li L, Mac-Mary S, Marsaut D, Sainthillier JM, Nouveau S, Gharbi T,et al. Age-related changes in skin topography and microcirculation. Arch Dermatol Res. 2006;297(9):412-6.
50. Girardeau S, Mine S, Pageon H, Asselineau D. The Caucasian and African skin types differ morphologically and functionally in their dermal component. Exp Dermatol. 2009;18(8):704-11.
1 - Doctor in Experimental Surgery - Associate professor at the Department of Surgery of the Federal University of Paraná, Curitiba, PR, Brazil
2 - Master in Surgery - Dermatologist at the Santa Casa de Misericórdia of Ponta Grossa, Ponta Grossa, PR, Brazil
3 - Master in Surgery - Plastic surgeon, Member of BSPS
4 - Plastic surgeon - Member of BSPS
5 - Physician - Resident in General Surgery, Angelina Caron Hospital, Campina Grande do Sul, PR, Brazil
6 - Master in Surgery - Professor of Pathological Anatomy at the State University of Ponta Grossa, Ponta Grossa, PR, Brazil
7 - Master in Surgery - Gynecologist and Obstetrician at the Santa Casa de Misericórdia of Ponta Grossa, Ponta Grossa, PR, Brazil
Institution: Pontifical Catholic University of Paraná.
Correspondent author:
Luiz Augusto Auersvald
Al. Presidente Taunay, 1756
Curitiba-PR - Brasil - CEP: 80430-000
E-mail: luizauersvald@uol.com.br
Article submitted: February 5, 2014
Article accepted: June 1, 2014


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter