

Original Article - Year 2014 - Volume 29 -
Influence of immunonutritional supplementation on skin wound healing in rats
Influência de dieta imunomoduladora na cicatrização cutânea em ratos
ABSTRACT
INTRODUCTION: The wound healing process is immediate and dynamic in order to restore anatomical and functional continuity, and there must be conditions for this process, which include a normal nutritional state. Among the existing supplemental formulas, immuno-enhancing diets have been proposed to improve the wound healing process and patients' clinical conditions. The influence of an immunomodulating diet (Impact®) on different variables of the skin healing process was evaluated.
METHOD: Healthy adult rats were randomly divided into four groups of diet supplementation or control. Two groups received their diets only pre-operatively while the other two groups received theirs perioperatively. Rats were subjected to three types of skin lesions. We evaluated the following aspects: changes in weight, development of raw areas, tensiometry of incisional wounds, re-epithelialization rates, and histological parameters.
RESULTS: There was no difference in weight changes. There was better closing rates of excisional wounds in groups supplemented with Impact® beginning on the fifth day after surgery (p = 0.02). The groups receiving the dietary supplements obtained the best results in tensiometry (p = 0.03), re-epithelialization rates (p = 0.04), differential cell counts (p < 0.001), and total amount of collagen (p < 0.001).
CONCLUSIONS: The study diet (Impact®) promoted better closure rates of raw wounds, faster re-epithelialization, scars with a greater tensile strength, and greater amounts of total collagen in wounds. There was no difference in any of the parameters analyzed compared with the groups supplemented with Impact® pre- and perioperatively.
Keywords: Immunomodulation; Immunonutrition; Wound healing; Immunomodulatory diet.
RESUMO
INTRODUÇÃO: O processo de cicatrização é imediato e dinâmico, com o objetivo de restaurar a continuidade anatômica e funcional, e devem existir condições para esse processo, o que inclui um estado nutricional adequado. Dentre as fórmulas de suplementação existentes, as imunomoduladoras têm sido implicadas na melhora do processo cicatricial e das condições clínicas dos pacientes tratados. Foi avaliada a influência da dieta imunomoduladora (Impact®) sobre diferentes variáveis do processo de cicatrização cutânea.
MÉTODO: Ratos adultos e nutridos foram divididos aleatoriamente em quatro grupos, a serem suplementados com a dieta em estudo e com a dieta controle. Dois grupos receberam as respectivas dietas apenas pré-operatoriamente e os outros dois grupos as receberam no perioperatório. Os ratos foram submetidos a três tipos de lesões cutâneas. Foram avaliados os seguintes aspectos: evolução dos pesos, evolução das áreas cruentas, tensiometria das feridas incisionais, taxas de reepitelização e parâmetros histológicos.
RESULTADOS: Não houve diferença na evolução dos pesos. Houve melhores índices de fechamento de feridas excisionais nos grupos suplementados com Impact®, a partir do quinto dia de pós-operatório (p=0,02). Os grupos suplementados com a dieta em estudo obtiveram melhores resultados em tensiometria (p = 0,03), taxas de reepitelização (0,04), contagem diferencial de células (p<0,001) e quantidade de colágeno total (p<0,001).
CONCLUSÕES: A dieta em estudo (Impact®) promove melhores taxas de fechamento de feridas cruentas, reepitelização mais rápida, cicatrizes com maior resistência tênsil e maiores quantidades de colágeno total nas feridas. Não houve diferença em nenhum dos parâmetros analisados em comparação dos grupos suplementados com Impact® pré e perioperatoriamente.
Palavras-chave: Imunomodulação; Imunonutrição; Cicatrização de feridas; Dieta imunomoduladora.
Skin wound healing is a dynamic process that involves a complex network of extracellular interactions, chemical mediators, and local inflammatory cells. The main objectives of this process are the restoration of tissue integrity and the maintenance of homeostasis1.
The wound healing process involves three sequential phases that are inter-related and occur in a dynamic and overlapping way: inflammatory, proliferative, and remodeling2.
For proper healing to occur, a number of conditions must be in place, including good tissue perfusion, the absence of debris, an efficient and fibroblast-rich immune system, and, in surgical patients, correct surgical technique3. Furthermore, adequate nutritional status is an important factor in achieving optimal wound healing process since tissue requires dynamic energy and plastic substrates provided by proteins and micronutrients4. Protein deficiency, potentially present in trauma conditions such as burns and major surgery, affect the healing process, favoring a higher risk of infection1,5,6. Physiologically, protein depletion in the wound healing process prolongs the inflammatory phase and impairs fibroplasia. Fibroblast proliferation, angiogenesis, and collagen production are decreased, and less tissue wound repair occurs as a result7.
In clinical practice, nutrients can be supplied via the oral, enteral, and parenteral routes. In recent years, there has been significant development of specialized nutritional formulas designed to meet the particular needs of organs and systems available in the form of oral supplements or formulas using enteral tubes8-11. Among these are immunomodulatory formulas that contain high levels of protein, are enriched with arginine and antioxidant nutrients such as zinc, selenium, or vitamins C and E, or are designed to meet the needs arising from the healing process and optimize its progress.
The use of immunonutrients in enteral formulas has been the subject of clinical and experimental studies. Nutritional therapy currently aims to provide energy and nutritional substrates and pharmacologically influence organic functions impaired by the patient's pathological state, particularly when immunosuppression and/or an exacerbated inflammatory state are present. Under these conditions, enteral or parenteral nutrient supplies with immunomodulatory functions are associated with benefits such as increased cellularity and the promotion of immune function7,12-14. Improving the immune system using nutrients such as n-3 fatty acids, arginine, and nucleotides has been linked to a reduced incidence of infectious complications, especially in patients undergoing surgery in the digestive tract for cancer and or those recovering from trauma6,15,16.
Considering that malnutrition may interfere with the healing process and that nutrients such as arginine and certain minerals and vitamins with known antioxidant effects can promote it, assessing the real impact of such nutrients on healing given different nutritional conditions is of particular interest, as is understanding certain cellular and molecular mechanisms involved in nutrition and wound healing. Provided that ethical concerns are addressed, experimental models are useful for wound healing research since they allow us to create conditions in a research laboratory that resemble those found in clinical practice.
The field of nutrition has changed profoundly since its inception. The original principle was to provide essential energy, protein, and micronutrients to compensate for a loss of muscle mass and prevent starvation-induced immune depletion. More recently, various dietary components have been used in an attempt to improve immune function, including specific amino acids such as arginine and glutamine, fatty acids (ω-3), and nucleic acids6. Among these substances, arginine supplementation is the most commonly researched compound and was identified as a modulator of wound healing in several experimental studies17. However, there is controversy in the literature and conflicting results. Furthermore, most studies examined the effects of immunonutrition only within the gastrointestinal tract18.
OBJECTIVE
The objective of this study was to study the effect of immunonutritional supplementation on different aspects of skin wound healing in rats. This study evaluates the influence of immunomodulating diet in its commercial form (Impact®) on different variables of the skin healing process, including the closure of excisional wounds, strength of scarring of incisional wounds, re-epithelialization of raw wounds and cell differentiation on scar tissue, and newly formed collagen matrix.
METHOD
This project was conducted in accordance with the ethical regulatory guidelines established by the Brazilian College of Animal Experimentation and approved by the Research Ethics Committee in the Health Science Center at the Federal University of Paraná, Curitiba, Brazil. The experiments were performed at the Laboratory of the Graduate Program in Surgery at the Federal University of Paraná under controlled temperature (22 ± 1ºC) and 12-h dark/light cycle conditions.
A total of 52 adult male Wistar rats (Rattus norvegicus albinus) (body weight, 230.08 ± 5.76 g) were allotted to four dietary supplementation groups: PI, ; PC, ; PPI, ; PPC, (n = 13 each). The rats were housed in groups of four to five animals. All groups were given ad libitum access to tap water and standard rat chow for 7 days prior to the start of the experiment and during the study regardless of whether oral supplementation with any immunonutritional formula (PI and PPI groups) or isocaloric and isoproteic control (PC and PPP groups) diet was given, both administered through an orogastric tube.
The rats were randomized to receive two different enteral formulas daily (Table 1). In the pre-operative groups (PI and PC), the rats received supplementation for only 7 days before surgery, whereas in the pre- and post-operative (PPI and PPC) groups, the rats received supplementation during the perioperative period (Figure 1) for a total of 14 days.


Figure 1. Description of Groups.
After 7 days of the pre-operative diet with supplementation, the rats were anesthetized and subjected to three dorsal wound excisions on full-thickness skin - one measuring 2 × 2 cm for evaluation of wound contraction and the other two measuring 6 mm in diameter for the study of re-epithelialization - and a ventral wound incision measuring 5 cm (Figure 2). The dorsal wounds were left open to heal spontaneously. The ventral wound was closed with simple mono nylon 5-0 stitches. On the eighth day after surgery, all rats were sacrificed and the dorsal lesions were excised and stored in saline solution and 10% formalin for histological analysis and tensile strength on hematoxylin-eosin staining and densitometry analysis of collagen.

Figure 2. Model of Incisional and Excisional Wounds.
Dorsal excisional wounds 2 × 2 cm were documented daily with standardized digital photography for further analysis of wound contraction rates with specific software (Figure 3). The excisional wound areas were calculated using ImageJ software (National Institutes of Health).
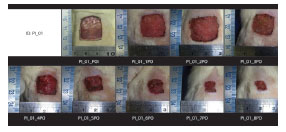
Figure 3. Changes in the Contraction Rates of Excisional Dorsal Wounds.
The collagen densitometry method was used to identify and quantify mature (type 1) and immature (type 3) collagen using the placement of Picrosirius Red on optical microscopy under polarized light and by performing image analysis in software (Image Pro-Plus 4.5; Midia Cibernetica). The percentages of collagen components shown in red and orange (type 1 collagen) and green (collagen type 3) were then calculated.
Both ends of the abdominal wound specimens were connected to a tensiometer (DL-500 MF; Emic) and distended at a constant speed (50 mm/min; load of 10 kg, 0.5% error) according to the technique proposed by Knolmayer et al19. Changes in tensile strength were continuously recorded on a computer and analyzed by software (Instron IX 7.26.00). Stretching was maintained until the specimen was completely broken as reflected by a sharp drop in the zero voltage curve (interruption). The maximum amount represented the total rupture strength. Thus, two voltage-related variables that reflect the strength of the anastomosis were obtained for each mouse.
Data were expressed as mean and standard deviation. Student's t-test and analysis of variance were used for group comparisons. Statistical significance was defined as the probability of an error of type <5%.
RESULTS
Body weight did not change significantly in any of the groups throughout the study (Figure 4). The excisional wound areas were assessed at different post-operative times. The area of the wound created by the initial procedure was similar in the control and study groups (PI = 4.52 cm2; PC = 4.30 cm2; PPI = 4.50 cm2; PPC = 4.25 cm2; p = 0.21). Evaluations of wound contraction rates showed a faster contraction in the groups receiving the immunomodulatory formula after the third post-operative day (Figure 5). The comparison between the two groups that received supplementation with the immunomodulatory formula showed no significant difference (p > 0.05).

Figure 4. Changes in Weight during the Experiment (in Grams).
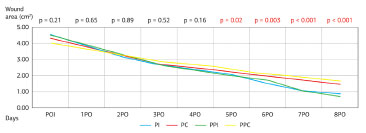
Figure 5. Wound Contraction Rate.
The tensile strength was examined and the groups receiving the immunomodulatory formula showed an increase of maximum tensile strength compared with the controls. Both groups showed a similar tensile strength (p > 0.05) (Figure 6).

Figure 6. Tensile Strength Rates in the Tissues.
Analysis revealed that re-epithelialization rates in the supplemented groups showed complete restoration of the epithelial layer on the seventh post-operative day in a larger number of cases (p = 0.04) compared with control group rats (Figure 7).
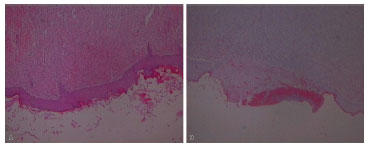
Figure 7. (A) Examples of Complete Histological Re-epithelialization and (B) Incomplete Epithelialization.
The analysis of collagen deposition in excisional wounds on the eighth day after surgery showed similar maturation rates (proportion of mature and immature collagen; p = 0.38). However, the groups supplemented with immunomodulatory formula had a greater total collagen deposition (p = 0.001).
DISCUSSION
Wound healing depends on a complex series of reactions and interactions of inflammatory mediators. Many intrinsic and extrinsic factors affect wound healing and some commercial options have been designed to neutralize negative interference in the healing process2,19,20. The interrelationship between nutrition and the process of wound healing, together with the immune system, has been the focus of increasing attention, and increasing numbers of amino acids and minerals have been identified as having immunomodulatory functions21.
The immune system can be subdivided into three sites of action that are potential targets for specific nutritional substrates: the function of the mucosal barrier, cellular defense functions, and local or systemic inflammation. The mucosal barrier was the first defense site considered for immunomodulatory therapy11. The second line of defense consists of cellular defense (macrophages, granulocytes, lymphocytes) and can be modulated by nutritional substrates to influence signal transduction. The essential component of the inflammatory immune response is represented by activation of the cascade systems (such as coagulant and complementary systems). Cytokines, eicosanoids, and platelet activating factors and nitric oxide (NO), as well as vasoactive amines, are some of the mediators involved22.
The inflammatory immune response can be regulated by nutritional substrates and their precursors, which are available in the oral supplementations. The immunomodulatory formulas use these different target sites of the immune system to control inflammation and enhance wound healing23. Glutamine, arginine, nucleotides, and polyunsaturated fatty acids (PUFAs) are considered of fundamental importance among all pharmacologically effective agents included as well as micronutrients such as zinc and various vitamins (A, C, E, and K).
Arginine is responsible for the modulation of various biological, immune, and metabolic functions. After trauma, for example, metabolism of essential amino acids increases12. In terms of bioavailability, catabolism does not provide sufficient amounts of arginine and NO, characteristic of severe trauma patients, which is the main reason for arginine supplementation in such situations24. Experimental studies suggest NO participation in the wound remodeling process13. Arginine acts as a substrate for protein synthesis, cell proliferation, neurotransmission, vasodilation, immunity, and wound healing. It is also a precursor of NO, urea, ornithine, proline, and other molecules and plays an important role in collagen synthesis25. Under stress, the metabolic demand for arginine increases, and supplementation is considered an adjunct support for wound healing8.
PUFAs changed the production of pro-inflammatory cytokines. N-3 fatty acids influence a number of inflammatory signal transduction processes for the expression of eicosapentaenoic acid and docosahexaenoic acid and compete with four series of leukotrienes for receptor occupancy26. Numerous studies have shown a correlation between increased levels of polyunsaturated ω-3 fatty acids and decreased production of proinflammatory cytokines in the plasma, but some studies have shown an increased production in some pro-inflammatory cytokines in cells such as peritoneal macrophages and fibroblasts treated with PUFA and ω-3.
The immunomodulatory formulas were identified as beneficial in wound healing in clinical studies5,15,16,18. However, the mechanisms responsible for this effect in tissue repair are not completely known. This study showed better wound contraction rates and collagen deposition in the groups receiving the immunomodulatory formula from the second day of supplementation onward, probably because of increased fibroblast function. Although the results of this study demonstrated several positive effects of nutritional immunomodulation, most cellular mechanisms remain unclear and require clarification to aid in our understanding of the influence of immunomodulatory formulas in the wound healing process.
CONCLUSION
The use of immunomodulatory formulas accelerates the wound healing process with increased collagen deposition and tissue tensile strength. The nutritional immunomodulation proved beneficial in several clinical trials in advance. However, the cellular mechanisms for improving wound healing processes remain unclear, so other experimental studies with immunomodulatory formulas are needed.
REFERENCES
1. Nguyen TT, Gilpin DA, Meyer NA, Herndon DN. Current treatment of severely burned patients. Ann Surg. 1996;223(1):14-25. http://dx.doi.org/10.1097/00000658-199601000-00004. PMid:8554414
2. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314-21. http://dx.doi.org/10.1038/nature07039. PMid:18480812
3. Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast. Reconstr. Surg. [Internet]. 2006; 117(Supl 7):1e-S-32e-S [cited 2013 Nov 8]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16801750.
4. Fürst P. Old and new substrates in clinical nutrition. J. Nutr. Am Soc Nutrition. 1998;128(5):789-96.
5. Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122(7):1763-70. http://dx.doi.org/10.1053/gast.2002.33587. PMid:12055582
6. McCowen KC, Bistrian BR. Immunonutrition: problematic or problem solving? Am J Clin Nutr. 2003;77(4):764-70. PMid:12663270.
7. Suchner U, Kuhn KS, Fürst P. The scientific basis of immunonutrition. Proc Nutr Soc. 2000;59(4):553-63. http://dx.doi.org/10.1017/S0029665100000793. PMid:11115790
8. Campos ACL, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care. 2008;11(3):281-8. http://dx.doi.org/10.1097/MCO.0b013e3282fbd35a. PMid:18403925
9. da Costa MAR, Campos ACL, Coelho JCU, de Barros AM, Matsumoto HM. Oral glutamine and the healing of colonic anastomoses in rats. JPEN J Parenter Enteral Nutr. 2003;27(3):182-56. http://dx.doi.org/10.1177/0148607103027003182. PMid:12757111
10. Debats IBJG, Wolfs TGAM, Gotoh T, Cleutjens JPM, Peutz- Kootstra CJ, van der Hulst RRWJ, et al. Role of arginine in superficial wound healing in man. Nitric Oxide. Elsevier Inc. 2009;21(3-4):175-83. http://dx.doi.org/10.1016/j.niox.2009.07.006.
11. Gardiner KR, Kirk SJ, Rowlands BJ. Novel substrates to maintain gut integrity. Nutr Res Rev. 1995;8(1):43-66. http://dx.doi.org/10.1079/NRR19950006. PMid:19094279
12. Stechmiller JK, Childress B, Cowan L. Arginine supplementation and wound healing. Nutr Clin Pract. 2005;20(1):52-61. http://dx.doi.org/10.1177/011542650502000152. PMid:16207646
13. Agren MS, Franzén L. Influence of zinc deficiency on breaking strength of 3-week-old skin incisions in the rat. Acta Chir Scand. 1990;156(10):667-70. PMid:2264423.
14. Shi HP, Efron DT, Most D, Tantry US, Barbul A. Supplemental dietary arginine enhances wound healing in normal but not inducible nitric oxide synthase knockout mice. Surgery. 2000;128(2):374-8. http://dx.doi.org/10.1067/msy.2000.107372. PMid:10923019
15. Braga M, Gianotti L, Cestari A, Vignali A, Pellegatta F, Dolci A, et al. Gut function and immune and inflammatory responses in patients perioperatively fed with supplemented enteral formulas. Arch Surg. 1996;131(12):1257-65. http://dx.doi.org/10.1001/archsurg.1996.01430240011001. PMid:8956766
16. Braga M, Gianotti L, Vignali A, Schmid A, Nespoli L, Di Carlo V. Hospital resources consumed for surgical morbidity: effects of preoperative arginine and omega-3 fatty acid supplementation on costs. Nutrition. 2005;21(11-12):1078-86. http://dx.doi.org/10.1016/j.nut.2005.05.003. PMid:16308130
17. Debats IBJG, Booi DI, Wehrens KME, Cleutjens J, Deutz NEP, van de Hogen E, et al. Oral arginine supplementation and the effect on skin graft donor sites: a randomized clinical pilot study. J Burn Care Res. 2009;30(3):417-26. http://dx.doi.org/10.1097/BCR.0b013e3181a28c15. PMid:19349894
18. Wu GH, Zhang YW, Wu ZH. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J Gastroenterol. 2001;7(3):357-62. PMid:11819790.
19. Knolmayer TJ, Cornell KM, Bowyer MW, McCullough JS, Koenig W. Imbrication versus excision for fascial healing. Am J Surg. 1996;172(5):506-11. http://dx.doi.org/10.1016/S0002-9610(96)00229-2. PMid:8942554
20. Coelho-Lemos ICM, Campos ACL, de Almeida M, Schüler SL, Gurmini J, Malafaia O, et al. In utero malnutrition influences wound healing of newborn rats as measured by tensile strength and collagen deposition. JPEN J Parenter Enteral Nutr. 2004;28(4):241-45. http://dx.doi.org/10.1177/0148607104028004241. PMid:15291405
21. Williams JZ, Abumrad N, Barbul A. Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg. 2002;236(3):369-75. http://dx.doi.org/10.1097/00000658-200209000-00013. PMid:12192323
22. Calder PC. Glutamine and the immune system. Clin Nutr. 1994;13(1):2-8. http://dx.doi.org/10.1016/0261-5614(94)90003-5. PMid:16843345
23. Raman M, Ambalam P, Kondepudi KK, Pithva S, Kothari C, Patel AT, et al. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes. Am Soc Nutrition. 2000;4(3):181-92.
24. Shi HP, Fishel RS, Efron DT, Williams JZ, Fishel MH, Barbul A. Effect of supplemental ornithine on wound healing. J Surg Res. 2002;106(2):299-302. http://dx.doi.org/10.1006/jsre.2002.6471. PMid:12175982
25. Morlion BJ, Stehle P, Wachtler P, Siedhoff HP, Köller M, König W, et al. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: a randomized, double-blind, controlled study. Ann Surg. 1998;227(2):302-8. http://dx.doi.org/10.1097/00000658-199802000-00022. PMid:9488531
26. Grimm H, Mayer K, Mayser P, Eigenbrodt E. Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br J Nutr. 2002;87(Supl 1):S59-67. http://dx.doi.org/10.1079/BJN2001457. PMid:11895155
1. PhD of Clinical Surgery at the Federal University of Paraná, Professor of Plastic and Reconstructive Surgery at the Federal University of Paraná, Curitiba, PR, Brazil
2. Head of Plastic and Reconstructive Surgery Services at the Federal University of Paraná, Associate Professor I of the Federal University of Paraná, Curitiba, PR, Brazil
3. Master of Clinical Surgery at the Federal University of Paraná, Doctor Surgeon of Digestive Devices, Curitiba, PR, Brazil
4. PhD and Master of Clinical Surgery at the Federal University of Paraná, Clinical Coordinator of the Multidisciplinary Nutritional Therapy Team, Oncology Research Center of Florianópolis, Curitiba, PR, Brazil
5. Master's Degree Student in Clinical Surgery at the Federal University of Paraná, General Surgery at Federal University of Paraná, Resident in Plastic and Reconstructive Surgery at the Clincial Hospital of Federal University of Paraná, Curitiba, PR, Brazil
6. Master and PhD in Clinical Surgery at the Federal University of Paraná, Professor and Coordinator of the Post-Graduate in Clinical Surgery at the Federal University of Paraná, Adjunct Professor in the Department of Nutrition at the Federal University of Paraná, Curitiba, PR, Brazil
Institution: Project carried out as part of the Graduate Program in Clinical Surgery at the Federal University of Paraná, Curitiba, PR, Brazil.
Corresponding author:
Maria Cecília Closs Ono
Federal University of Paraná, Plastic and Reconstructive Surgery Alto da Glória
Rua General Carneiro, 181, 9º Andar
Curitiba, PR, Brazil CEP 80060-900
E-mail: mccono@gmail.com
Article received: March 10, 2014.
Article accepted: August 3, 2014.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter