

Original Article - Year 2014 - Volume 29 -
Abdominal wall repair with double-mesh polypropylene/polyglecaprone after TRAM flap surgery for breast reconstruction
Reparação da parede abdominal com tela dupla de polipropileno e poliglecaprone pós-retalho TRAM em reconstrução mamária
ABSTRACT
INTRODUCTION: The repair of the abdominal wall after breast reconstruction with a transverse rectus myocutaneous (TRAM) flap is a challenge for the surgeon, and there is still no consensus in the literature about which is the best technique. The objective of this study is to evaluate the efficiency of the Ultrapro® mesh in two different anatomical planes for the repair of the abdominal wall after TRAM flap surgery.
METHOD: This is a retrospective study conducted through a medical records review of 24 patients who underwent breast reconstruction with a pedicle TRAM flap, and repair of abdominal donor site with a dual mesh of polypropylene and polyglecaprone - Ultrapro, at the Plastic Surgery Division of the Clinics Hospital of the Medicine Faculty of Ribeirão Preto - University of São Paulo. We evaluated the risk factors for abdominal hernias or bulges, time of breast reconstruction, postoperative complications (including abdominal hernias or bulges), and postoperative follow-up.
RESULTS: Of the 24 patients with a mean age of 51 years, 10 (41.6%) had a comorbidity. In 95.8% of the patients, breast reconstruction was late; the TRAM flap was a single pedicle in 58.4% of cases. The most frequent postoperative complications were suture dehiscence (25%) and seroma (21%). Two patients (8.4%) were found to have abdominal hernia, and three patients (12.5%) had abdominal bulging. The postoperative follow-up ranged from 5 to 48 months (average, 23.4 months, SD = 13.28).
CONCLUSION: The use of the Ultrapro hybrid mesh at two anatomical planes proved to be an alternative for the repair of the abdominal wall after TRAM flap surgery for breast reconstruction, with low morbidity of the abdominal donor site and complication rates similar to literature data.
Keywords: Breast reconstruction; Abdominal hernia; Synthetic mesh; TRAM flap; Mastectomy.
RESUMO
INTRODUÇÃO: A reparação da parede abdominal após reconstrução mamária com retalho TRAM representa um desafio para o cirurgião, ainda sem consenso na literatura em relação à melhor técnica. O objetivo deste estudo foi avaliar a eficiência da tela Ultrapro® em dois planos anatômicos distintos para reparação da parede abdominal pós-retalho TRAM.
MÉTODO: Um estudo retrospectivo foi realizado por meio da revisão de prontuários de 24 pacientes submetidas à reconstrução de mama com retalho TRAM pediculado e reparo da área doadora abdominal com tela dupla de polipropileno e poliglecaprone - Ultrapro® pela Divisão de Cirurgia Plástica do HCFMRP-USP. Foram avaliados fatores de risco para hérnias ou abaulamentos abdominais, momento da reconstrução de mama; complicações pós-operatórias, incluindo hérnias ou abaulamentos abdominais, e tempo de seguimento pós-operatório.
RESULTADOS: Do total de 24 pacientes com idade média de 51 anos, 10 (41,6%) apresentavam alguma comorbidade. Em 95,8% das pacientes a reconstrução mamária foi tardia e o retalho TRAM foi unipediculado em 58,4% dos casos. As complicações pós-operatórias mais frequentes foram deiscência de sutura (25%) e seroma (21%). Duas pacientes (8,4%) tiveram diagnóstico de hérnia abdominal e três pacientes (12,5%) apresentaram abaulamento abdominal. O tempo de seguimento pós-operatório variou de 5 a 48 meses (média 23,4 meses, DP: 13,28).
CONCLUSÃO: O uso da tela híbrida Ultrapro® em dois planos anatômicos demonstrou ser mais uma alternativa para o reparo da parede abdominal pós retalho TRAM em reconstrução mamária, com baixa morbidade da área doadora abdominal e índices de complicações semelhantes aos dados da literatura.
Palavras-chave: Reconstrução mamária; Hérnia abdominal; Tela sintética; Retalho TRAM; Mastectomia.
Initially described by Holmström in 19791 and popularized by Hartrampf et al. in 19822, the use of the transverse rectus myocutaneous (TRAM) flap has spread widely over the past decades and is considered the gold standard for breast reconstruction with autologous tissues, owing to its excellent and long-lasting aesthetic results.
However, the evolution of breast reconstruction techniques did not completely eliminate donor site morbidity, such as the development of hernias, bulges, and asymmetries in the body contour. In this context, abdominal wall repair is a challenge for the surgeon, and there is still no consensus in the literature about which is the best technique3,4. The treatment alternatives for the TRAM flap donor site include preserving the structures of the abdominal wall, local flaps, and the use of synthetic fabrics in one or more layers5.
Currently, numerous types of mesh are commercially available, and they differ in material, texture, pore size, weight, elasticity, tissue reaction, biocompatibility, and absorption6-8. Several studies were conducted to evaluate the ideal mesh and the best implantation technique9-11. The ideal mesh should restore abdominal function; physiologically integrate into the abdominal wall with maximum biocompatibility, minimizing complications such as infection, chronic pain, bulging, and hernias; and be easily manageable.
The marketed meshes can be classified into heavy-weight meshes with reduced pores and lightweight meshes with large pores >1mm, 20-35% elasticity, and tensile strength of at least 16 N/cm, in addition to hybrid meshes with absorbable and nonabsorbable components.
Recent studies indicate that lightweight porous meshes were designed to mimic the tensile strength of the abdominal wall, be physiologically adapted to local tissues, and have less contact surface, which reduces foreign body reactions and allows the formation of more flexible scars in the long term. A similar elasticity to that of the abdominal wall ensures better quality and physiological repair, providing even greater comfort to the patient12.
The dual polypropylene and polyglecaprone mesh Ultrapro® consists of a lightweight mesh and large pores, >3 mm, with an absorbable component - Monocryl® (polyglecaprone 25) and a nonabsorbable component - Prolene® (polypropylene). Clinical studies demonstrate satisfactory and encouraging results with the use of this material in hernia repair12; however, after an extensive search of the PubMed-Medline database, we found no reports on the use of a hybrid dual mesh of polypropylene and polyglecaprone in abdominal wall repair after TRAM flap surgery.
OBJECTIVE
The objective of this study is to evaluate the efficiency of the hybrid dual mesh of polypropylene and polyglecaprone at two different anatomical planes, for the repair of the abdominal wall after TRAM flap surgery in patients undergoing breast reconstruction.
METHOD
A retrospective study was conducted through a medical records review of 24 patients who underwent immediate or delayed breast reconstruction with pedicle TRAM, and repair of the abdominal donor site with a dual mesh of polypropylene and polyglecaprone (Ultrapro). The patients were operated at the Plastic Surgery Division of the Clinics Hospital of the Medicine Faculty of Ribeirão Preto, University of São Paulo (CHMFRP-USP) from March 2008 to November 2012. The study was approved by the ethics committee for research of this institution.
We evaluated the risk factors for abdominal hernias or bulges, such as obesity (body mass index [BMI] >30 kg/m2), comorbidities (diabetes mellitus, smoking, chronic obstructive pulmonary disease, intestinal constipation, or malnutrition), and previous abdominal surgical scars. We further evaluated the time of breast reconstruction (immediate or delayed); presence or absence of neoadjuvant and adjuvant treatment; difficulties or postoperative complications, including the development of hernias and abdominal bulges; operative time, duration of hospital stay, and duration of drain; postoperative follow-up time; and need for additional surgical procedures at the abdominal donor site. Finally, we evaluated the documentation of medical records concerning the degree of satisfaction reported by patients on the aesthetic and functional aspects, considering the impact on their daily activities.
Abdominal ultrasound was used to confirm any clinical suspicion of hernia or bulging.
Surgical Technique Description
The abdominal wall repair after TRAM, standardized by the Plastic Surgery Division of the CHMFRP-USP and conducted in 24 patients in this study, consists of the approximation of the anterior aponeurosis of the rectus abdominis muscle with simple stitches with mononylon 2-0, leaving only the defect for later mesh wall reinforcing. Then, fixation of polypropylene and polyglecaprone mesh (Ultrapro-Ethicon, Bridgewater, NJ, USA) at two anatomical planes is done.
The first mesh, rectangular in shape when the TRAM flap is monopedicle, is allocated to the donor site of the rectus abdominis muscle in the longitudinal direction, in the space between the anterior and rear aponeurosis (Figure 2).
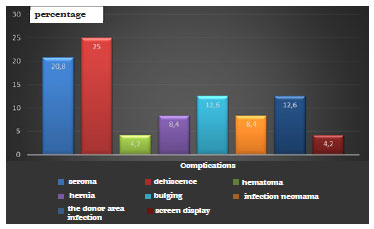
Figure 1 - Percentage distribution of postoperative complications.
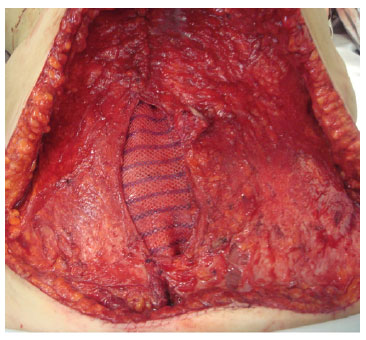
Figure 2. Intraoperative photograph showing the first mesh positioned at the donor site of the right rectus abdominis muscle.
When the bipedicle TRAM flap is used, the mesh is placed in the same space as the letter "H" shape. A second, wider, mesh is positioned in the lower abdomen on the anterior aponeurosis and the area without it (due to its inclusion in the flap), covering the first mesh. Next, fixation is conducted with simple nylon 2-0 points on the alba line, laterally in the joint tendon area and at the junction of the lateral border of the rectus abdominis, and the medial border of the external oblique muscle (Figure 3).
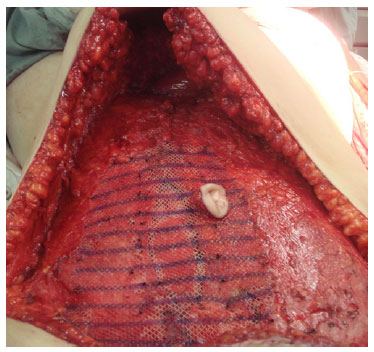
Figure 3. Intraoperative photograph showing the second mesh positioned and fixed on the previous aponeurosis and the defect area.
RESULTS
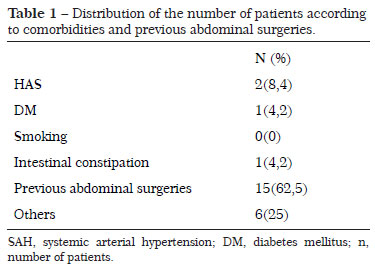
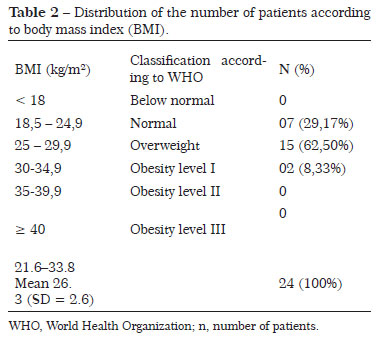
Of the 24 patients (mean age, 51 years), 10 (41.6%) had a comorbidity (Table 1) and 15 (62.5%) had previous abdominal scars (Pfannenstiel, McBurney, and medians). No patient was a smoker and/or malnourished. The BMI ranged from 21.6 to 33.8 kg/m2, with an average of 26.3 ± 2.6 kg/m2. Two patients (8.33%) had a BMI >30 kg/m2 (Table 2).
In 15 patients (62.5%), the mastectomy was on the left. Eleven patients (45.8%) underwent hormone therapy, 15 (62.5%) chemotherapy, and 17 (62.5%) radiotherapy at some time during the treatment.
There was a predominance of delayed breast reconstruction (95.8%). In 4 patients (16.7%), the TRAM flap was single pedicle ipsilateral; in 10 patients (41.7%), contralateral single pedicle; and in 10 patients (41.7%), double pedicle. In 15 patients (62.5%), previous surgeries were performed with flap empowerment.
The mean operative time was 4.6 h (range, 2.5-8 h). The hospital stay ranged from 2 to 8 days, with an average of 4 days. The abdominal drain duration ranged from 4 to 21 days, with an average time of 8 days.
The most frequent postoperative complications were suture dehiscence (25%) and seroma (21%), as shown in Figure 1.
Two patients (8.4%) had a clinical and radiological diagnosis of abdominal hernia, one in the 4th postoperative month and another on the 14th month; both of these patients had delayed breast reconstruction with contralateral single-pedicle TRAM flaps. Three patients (12.5%) had abdominal bulging diagnosed in the 5th, 12th, and 20th postoperative month. One of them underwent breast reconstruction with double-pedicle TRAM flap, and the other with contralateral single-pedicle TRAM.
In four cases (16.7%), complementary surgical procedures were performed at the abdominal donor site, with two hernioplasty (8.4%), correction of abdominal bulging, and partial withdrawal of the infected mesh, with wall resuture.
The postoperative follow-up ranged from 5 to 48 months (mean, 23.4 ± 13.28 months). Seventy-nine percent of the patients considered the aesthetic results as optimal, 16% as good, 4.2% as regular, and none considered the results as bad.
DISCUSSION
The TRAM flap is considered the gold standard in postmastectomy breast reconstruction. It has received wide acceptance among surgeons because it promotes excellent and long-lasting aesthetic results in one procedure, without the need for breast implants. However, the morbidity involving the abdominal donor site is still a concern. Kroll and Marchi13 reported weakness in the abdominal wall in >40% of patients, and the incidence of abdominal contour change reaches 16.7% according Souto et al.14.
The reconstruction should restore the structural and functional integrity of the abdominal wall, in a stable way and without tension. The use of synthetic mesh in the reinforcement of the abdominal wall reduces the incidence of complications such as hernias and bulges3, 15,16.
Several synthetic meshes are marketed, and they differ in material, texture, pore size, elasticity, tissue reaction, biocompatibility, and absorption. The individual response to each type of mesh is variable, and this can contribute to various complications such as seroma, chronic pain, or infections17.
The first-generation classic meshes have a high density and reduced pores. The second-generation meshes, among them polypropylene and polyglecaprone (Ultrapro), mimic the physiological properties of the abdominal wall: they have better elasticity, confer comfort in the abdominal donor site, and result in lower foreign body reaction and more flexible scars, with a positive impact on the quality of life of the patients. Those meshes are also related to lower inflammatory response, lower incidence of chronic pain, and lower recurrence rates in the case of hernias. There are few clinical studies on the use of this material in the treatment of hernias, despite the reported satisfactory results12. There are no reports on the use of the hybrid dual mesh of polypropylene and polyglecaprone in the repair of the abdominal wall after TRAM flap surgery.
According to the literature, the incidence of hernias and bulges after breast reconstruction with TRAM is about 10% with nonabsorbable mesh16-19 and 55% with the use of Parietex Progrip mesh in a unique anatomical plane20. Souto et al.14 reported a reduction of abdominal deformities with the dual mesh of polypropylene, obtaining an incidence of hernias and bulges of 16.7%. In this study, by using a hybrid dual mesh of polypropylene and polyglecaprone (Ultrapro) for abdominal wall repair after TRAM, we observed an incidence of 8.4% hernia and 12.5% bulging.
Also, the incidences of infection (12.5%) and mesh exposure (4.2%) in this study were similar to those described in the literature, ranging from 0% to 11.8% and 1.5% to 4%, respectively15,16,19.
CONCLUSION
The use of a polypropylene and polyglecaprone hybrid mesh (Ultrapro) at two anatomical planes proved to be an alternative for abdominal wall repair after TRAM flap breast reconstruction, with low morbidity and providing comfort in the abdominal donor site. The complication rates were similar to the literature data. However, further studies are needed to confirm its wider applicability in strengthening the abdominal wall after TRAM surgery.
REFERENCES
1. Holmström H. The free abdominoplasty flap and its use in breast reconstruction: an experimental study and clinical case report. Scand J Plast Reconstr Surg. 1979;13:423-7.
2. Hartrampf CR Jr, Scheflan M, Black P. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69:216-25.
3. Kroll SS, Schusterman MA, Reece GP, Miler MJ, Geofrey R, Evans G. Abdominal wall strength, bulging and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg. 1995;96(3):616-9.
4. Rossetto LA, Abla LEF, Vidal R, Garcia EB, Gonzalez RJ, Gebrim LH, et al. Factors associated with hernia and bulge formation at the donor site of the pedicled TRAM flap. Eur J Plast Surg. 2010;33:203-8.
5. Oliveira Jr FC, Melega JM, Pinheiro AS, Destro C, Maciel PJ. Comparação entre a utilização da tela de Marlex em um e dois planos para a reconstrução da parede abdominal pós-TRAM. Tórax e Tronco. 2010; Suppl 25(3):49.
6. Schug-Paß C, Tamme C, Sommerer F, Lippert H, Köckerling F. A lightweight, partially absorbable mesh (Ultrapro) for endoscopic hernia repair: experimental biocompatibility results obtained in aporcine model. Surg Endosc.2007;22:1100-6.
7. Junge K, Rosch R, Krones CJ, Klinge U, Mertens PR, Lynen P, et al. Influence of polyglecaprone 25 (Monocryl) supplementation on biocompatibility of a polypropylene mesh for hernia repair. Hernia. 2005;9:212-7.
8. SchugPaß C, Tamme C, Köckerling F. A lightweight polypropylene mesh (TiMesh) for laparoscopic intraperitoneal repair of abdominal wall hernias: comparison of biocompatibility with the dual mesh in an experimental study using the porcine model. Surg Endosc. 2006;20:402-9.
9. Weyhe D, Belyaev O, Müller C, Meurer K, Bauer KH, Papapostolou G, Uhl W. Improving outcomes in hernia repair by the use of light meshes - a comparison of different implant constructions based on a critical appraisal of the literature. World J Surg. 2007;31:234-44.
10. Klosterhalfen B, Klinge U, Schumpelick V. Functional and morphological evaluation of different polypropylene-mesh modifications for abdominal wall repair. Biomaterials.1998;19:2235-46.
11. Klosterhalfen B, Junge K, Klinge U: The lightweight and large porous mesh concept for hernia repair. Expert Rev Med Device. 2005;2:103-17.
12. Holzheimer RG. First results of Lichtenstein hernia repair with Ultrapro-mesh as cost saving procedure-quality control combined with a modified quality of life questionnaire (SF-36) in a series of ambulatory operated patients. Eur J Med Res. 2004;9:323-7.
13. Kroll SS, Marchi M. Comparison of strategies for preventing abdominal-wall weakness after TRAM flap breast reconstruction. Plast Reconstr Surg. 1992;89:1045.
14. Souto LRM, Cardoso LAA, Claro BM, Peres MAO. Double-mesh technique for correction of abdominal hernia following mammary reconstruction carried out with bipedicled TRAM flap and the primary closing of the donor area by using a single polypropylene mesh. Aesth Plast Surg. 2001;35:184-91.
15. Zienowicz RJ, May JW Jr. Hernia prevention and aesthetic contouring of the abdomen following TRAM flap breast reconstruction by the use of polypropylene mesh. Plast Reconstr Surg. 1995;96:1346.
16. Moscona RA, Ramon U, Toledano H, Barzilay G. Use of synthetic mesh for the entire abdominal wall after TRAM flap transfer. Plast Reconstr Surg.1998;101:706-10.
17. Seiler C, Baumann P, Kienle P, Kuthe A, Kuhlgatz J, Engemann R, et al. A randomised, multi-centre, prospective, double blind pilot-study to evaluate safety and efficacy of the non-absorbable Optilene® Mesh Elastic versus the partly absorbable Ultrapro® Mesh for incisional hernia repair. BMC Surg. 2010;10:21.
18. Ramirez OM, Ruas E, Dellon AL. Components separation method for closure of abdominal wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519-26.
19. Kroll SS, Netscher DT. Complications of TRAM flap breast reconstruction in obese patients. Plast Reconstr Surg. 1989;84(6):886-92.
20. Svaerdborg M, Damsgaard TE. Donor-site morbidity after pedicled TRAM-Flap breast reconstruction: a comparison of two different types of mesh. Ann Plast Surg. 2013;71(5):476-80.
1. Medical Resident - Medical Doctor Resident of the Second Year of Plastic Surgery of the Clinics Hospital of the Medicine Faculty of Ribeirão Preto - University of São Paulo (CHMFRP-USP)
2. Medical Resident - Medical Resident of the Second Year of Plastic Surgery of the CHMFRP-USP
3. Medical Resident - Medical Resident of the Second Year of Plastic Surgery of the CHMFRP-USP
4. Medical Assistant - Medical Assistant of the Surgery Division of Plastic Surgery of the CHMFRP-USP
5. Doctor, Full Member of BSPS and BCS - Chief of the Plastic Surgery Division of the CHMFRP-USP
Institution: Clinics Hospital of the Medicine Faculty of Ribeirão Preto - University of São Paulo (CHMFRP-USP).
Corresponding Author:
Prof. Dr. Jayme Adriano Farina Jr
Department of Surgery and Anatomy, Division of Plastic Surgery, Medicine Faculty of Ribeirão Preto, University of São Paulo
Avenida Bandeirantes, 3900 - 9º andar - Monte Alegre
Ribeirão Preto, SP, Brazil - Zip code: 14048-900
E-mail: jafarinajr@fmrp.usp.br
Article received: January 2, 2014.
Article accepted: August 31, 2014.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter