

Original Article - Year 2015 - Volume 30 -
Immediate breast reconstruction with latissimus dorsi flap and silicone implant
Reconstrução mamária imediata com retalho do músculo grande dorsal e implante de silicone
ABSTRACT
INTRODUCTION: The first effective breast cancer treatment was described in 1894. Less aggressive surgeries were developed in the 1960s and 70s, without increased mortality due to cancer. With similar historical evolution, the latissimus dorsi muscle flap (LDMF) procedure was introduced in 1906. Seventy years after its first description, LDMF gained popularity as an option for breast reconstruction.
METHOD: A retrospective clinical study was conducted using data obtained from 22 patients undergoing immediate breast reconstruction with LDMF and silicone implants between February 2012 and December 2013.
RESULTS: No latissimus dorsi flap necrosis or breast reconstruction losses were observed in this study. Ten cases (45%) of seroma were detected in the dorsal region, three cases (14%) of partial necrosis of the mastectomized skin, and three cases (14%) of partial surgical wound dehiscence. Statistical significant risk factors for the complications observed have not been emphasized. There were four cases (18.18%) of muscle and skin atrophy associated with implants, and two cases (9.09%) of capsular contracture. Only one case was not associated with radiotherapy. However, there were no statistically significant differences in adjuvant radiotherapy and late complications (p = 0.635).
CONCLUSION: LDMF associated with silicone implants is a safe and reliable option for immediate breast reconstruction after mastectomies.
Keywords: Breast/surgery; Breast neoplasms; Surgical flaps; Breast implant.
RESUMO
INTRODUÇÃO: O primeiro tratamento eficaz para o câncer de mama foi descrito em 1894. A partir das décadas de 60 e 70, cirurgias menos agressivas foram desenvolvidas, sem prejuízos oncológicos. Com evolução histórica semelhante, o retalho do músculo grande dorsal (RMGD) foi introduzido em 1906. Contudo, apenas 70 anos após sua primeira descrição, ele ganhou popularidade como uma opção para as reconstruções mamárias.
MÉTODO: Estudo clínico retrospectivo realizado por meio da coleta de dados de 22 pacientes submetidas à reconstrução mamária imediata com emprego do RMGD associado a implante de silicone durante o período de fevereiro de 2012 a dezembro de 2013.
RESULTADOS: Não houve necrose do retalho de grande dorsal ou perda da reconstrução mamária nos casos estudados. Foram observados 10 casos (45%) de seroma em região dorsal, 3 casos (14%) de necrose parcial da pele da mastectomia e 3 casos (14%) de deiscência parcial da ferida operatória. Não foram evidenciados fatores de risco com significância estatística para as complicações apresentadas. Ocorreram 4 casos (18,18%) de alterações de cobertura do implante, com atrofia muscular e cutânea, e 2 casos (9,09%) de contratura capsular. Apenas um caso não foi associado à radioterapia. Contudo, não houve significância estatística em relação à radioterapia adjuvante e às complicações tardias apresentadas (p = 0,635).
CONCLUSÃO: O RMGD associado ao implante de silicone é uma opção segura e confiável para a reconstrução mamária imediata após mastectomias.
Palavras-chave: Mama/cirurgia; Neoplasias da mama; Retalhos cirúrgicos; Implante mamário.
Breast cancer is the most common cancer among women. The National Cancer Institute (INCA) estimated 57,120 new cases in Brazil in 2014. Statistical analyses indicate increased incidence of cancer in developed and developing countries. Mortality rates in Brazil remain high, as the disease is still often diagnosed in advanced stages1.
The first effective breast cancer treatment was classical radical mastectomy, described by Halsted in 18942. In the 1960s and 70s, less aggressive surgical techniques were developed to reduce surgical morbidity and avoid prejudicing the results obtained with the cancer treatment3-6. Preservation of mastectomized skin and, when possible, the nipple-papillary complex (CPC), are current treatment options that provide similar therapeutic results7-9. Consequently, development of reconstructive options to repair increasingly smaller mammary sequelae has accompanied these improvements10.
With a similar historical evolution, Tansini first described the latissimus dorsi muscle flap procedure in 190611. However, not until 1976 did Olivari suggest its usefulness to cover radiotherapy-induced injuries of the chest wall12. The following year, Schneider et al.13 introduced the myocutaneous flap in the latissimus dorsi muscle (LDMF) island for breast reconstruction; in 1978, Bostwick et al. 14 described a modified technique in which the pectoralis major muscle was used to cover the breast implant.
Since that time, LDMF has gained popularity as a relatively simple procedure for introducing a skin island with significant vascularization15.
OBJECTIVE
This study assessed surgical results of immediate breast reconstruction with LDMF associated with silicone implants.
METHOD
This observational, retrospective, and clinical study reviewed medical records of patients who underwent surgeries and were monitored on an outpatient basis. Data were collected from 22 patients who received immediate breast reconstruction with LDMF associated with cohesive gel anatomical silicone implants between February 2012 and December 2013.
Surgical procedure
Surgical plans for accessing and performing oncologic resections is were developed and performed by the mastology team. The dorsal skin island has a horizontal orientation, with a size and location unique for each case (Figures 1 and 2).
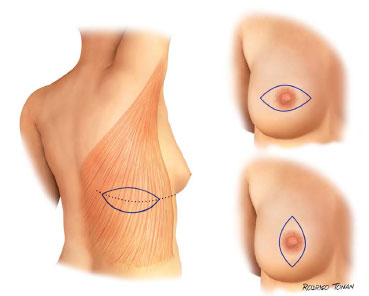
Figure 1. Standard surgical schedules.
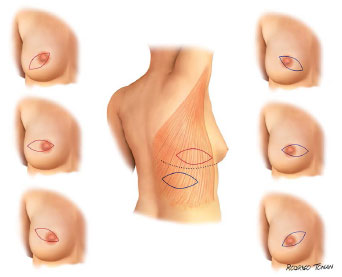
Figure 2. Standard surgical schedule variants.
After cancer resection, the patient was positioned in lateral decubitus. The muscle was entirely dissected, keeping the areolar layer of subcutaneous tissue in its previous face (Figure 3).
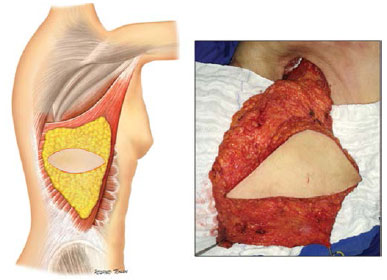
Figure 3. Surgical anatomy of the latissimus dorsi.
Transposition of the muscle to the affected hemithorax was performed by tunneling the upper third of the lateral chest area to preserve the inferolateral contour of the breast.
The muscle was then fixed peripherally to cover the entire mastectomized area, thus delimiting the implant region (Figure 4).
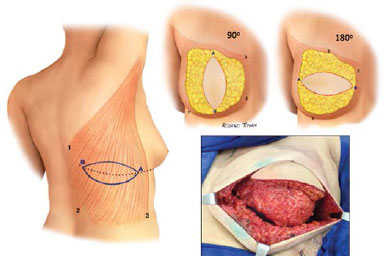
Figure 4. Variations in flap position in reconstructed breasts.
Implant selection was multifactorial, based on criteria such as the base diameter of the delimited region in the hemithorax, the weight of the resected breast, and the amount of remaining tissue. The purpose was to comfortably cover the implant without restriction, aiming for maximum projection and ptosis of the reconstructed breast.
If necessary, cutaneous adjustments of the mastectomized skin were performed and the skin island of the flap positioned to optimize its exposure and location within the reconstructed breast.
Two counter-opening suction drains were inserted, one in the dorsal region and the other in the breast, above the muscular plane.
The suture was performed by planes with polyglactin 2.0, 3.0 and poliglecaprone 25 4.0. Adhesion points were not used in the area detached from the dorsal region.
Patients were maintained in chest compressive wraps for 24 hours. The wraps were subsequently replaced with a surgical bra, which was maintained for 30 days. In addition, 500 mg cefadroxil were prescribed every 12 hours for 14 days. The breast drain was removed after approximately seven days and the dorsal almost 14 days later, when the fluid outflow fell below 30 mL over 24 hours.
All patients were postoperatively monitored on an outpatient basis and their progress followed by photographic documentation.
Statistical analyses were performed on the collected data.
RESULTS
The average patient age in this study was 44 years and 2 months ± 8.72, ranging from 30 to 71 years. The median age was 42 years and 6 months.
The average body mass index (BMI) was 25.7 ± 4.17 kg/m2, ranging from 37.7 to 20.3 kg/m2. The median BMI was 25.2 kg/m2.
Among 22 patients analyzed in this study, one (4.5%) had hypertension, one (4.5%) had diabetes mellitus, and four (18.2%) were smokers at the time of surgery.
The average length of hospitalization was 2 ± 0.4 days, ranging from 1 to 3 days. The median was 2 days.
Eleven patients (50%) underwent Madden modified radical mastectomy, nine (41%) underwent simple mastectomy and sentinel lymph node biopsy (LNB), one (4.5%) patient received adenectomy and LNB, and one patient (4.5%) underwent only adenectomy.
Anatomopathological diagnoses of the tumors included invasive ductal carcinoma (IDC) in 19 patients (86%), invasive lobular carcinoma (ILC) in two patients (9.1%), and in situ ductal carcinoma (ISDC) in one patient (4.5%). The average tumor diameter was 35 ± 28.4 mm, ranging from 10 to 140 mm. The median tumor diameter was 27 mm.
Among patients analyzed in this study, one (4.5%) was stage zero, six (27.3%) were stage IA, seven (31.8%) were stage IIA, two (9.1%) were stage IIB, three (13.6%) were stage IIIA, one (4.5%) was stage IIIB, and three (9.1%) were stage IIIC.
The clinical treatments included adjuvant chemotherapy (12 patients, 54.5%), neoadjuvant chemotherapy (7 patients, 31.8%), and no chemotherapy (3 patients, 13.6%). Adjuvant radiotherapy was performed in 11 (50%) of the 22 patients.
The average surgical procedure duration was 5 hours and 48 minutes ± 1 hour and 6 minutes, ranging from 3.5 to 8 hours. The median surgical time was 5 hours and 45 minutes.
The average specimen weight was 493 ± 241 grams, ranging from 90 to 918 grams. The median weight was 518 grams.
All procedures were performed using anatomically shaped silicone implants with maximum projection. The average weight of the implants was 373 ± 100 grams, varying from 165 to 510 grams. The median weight was 385 grams.
The average resected breast skin defect area was 49 ± 27.5 cm2, ranging from 0 to 117.8 cm2. The median was 43.6 cm2.
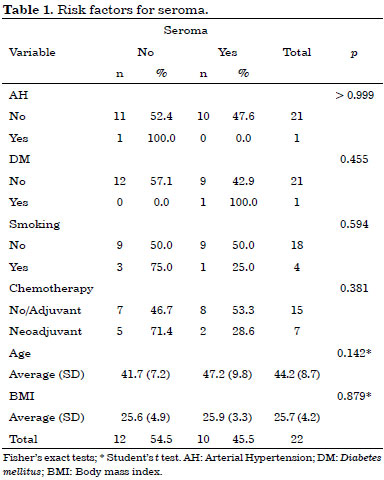
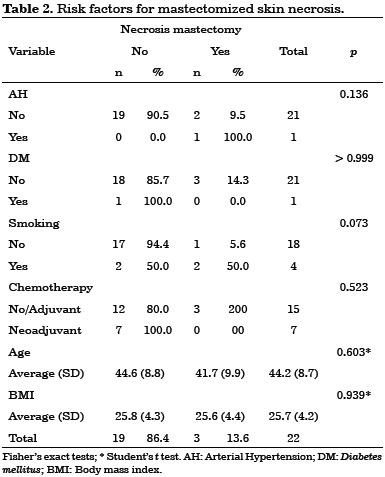
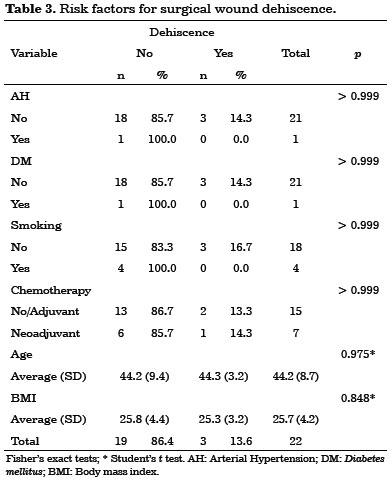
Early complications occurred within 30 days after surgery. They included 10 cases (45%) of seroma in the dorsal region, three cases (14%) of partial necrosis of the mastectomized skin, and three cases (14%) of partial surgical wound dehiscence. No latissimus dorsi flap necrosis was observed in this study.
No statistically significant risk factors were associated with early complications (Tables 1, 2 and 3).
All early complications were treated on an outpatient basis, without hospitalization.
Late complications were defined as those that occurred more than 30 days after surgery. Were observed four cases (18.18%) of changes of implant coverage with muscle and skin atrophy, predominantly in the upper pole of the breast. Only one case was not associated with radiotherapy. All cases were treated with fat grafts. We also observed two cases (9.09%) of capsular contracture. Both were associated with radiotherapy and required surgical capsulotomy (Figure 5).
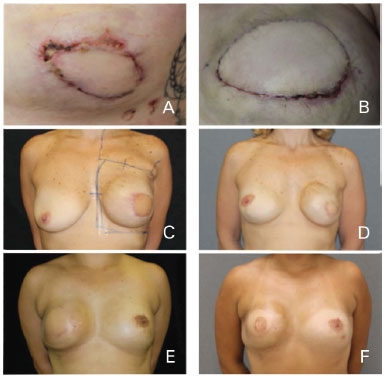
Figure 5. Complications. (A, B) Skin necrosis of the mastectomy site. (C, D, E, F) Capsular contracture and atrophy after radiotherapy.
There were no statistically significant relationships between adjuvant radiotherapy and late complications (Table 4).
No clinical complications were observed in the patients evaluated in this study.
The average patient follow-up period was 16 months and 24 days ± 7 months and 12 days, ranging from 27 months and 27 days to 5 months and 27 days. The median was 17 months and 3 days (Figures 6, 7, 8, 9 and 10).
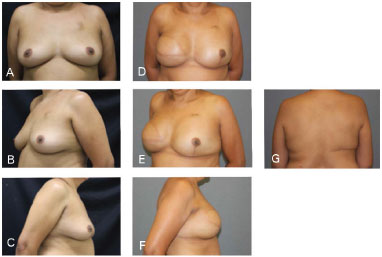
Figure 6. Clinical case 1: Classical mastectomy with 435 g anatomical implant and left adenectomy (400 g). A, B, and C: Preoperative; D, E, F, and G: Postoperative follow-up at 18 months.
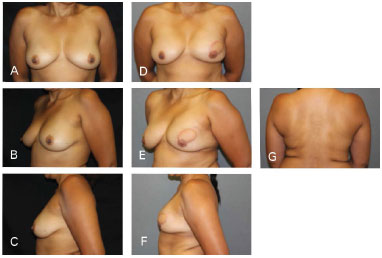
Figure 7. Clinical case 2: Mastectomy that preserved the skin, using a 335 g anatomical implant, Rec. NAC (contralateral inguinal and nipple). A, B and C: Preoperative; D, E, F, and G: Postoperative follow-up at 18 months.
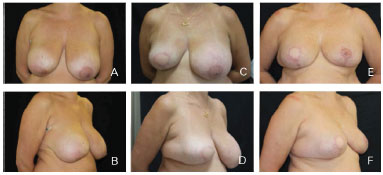
Figure 8. Clinical case 3; Mastectomy to D. The reconstruction involved "T"-shaped adjustments, a 550 g anatomical implant, and symmetrization with mammoplasty. A and B: Preoperative; C and D: Postoperative follow-up at 6 months; E and F: Postoperative follow-up at 12 months.
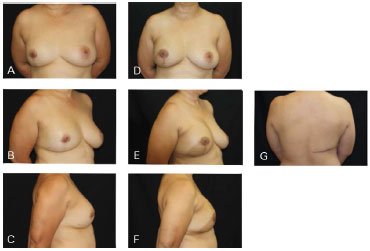
Figure 9. Clinical case 4: Mastectomy with supratumoral skin resection with post-QUART recurrence. A 310 g anatomical implant was used, along with NAC preservation. A, B and C: Preoperative; D, E, F, and G: Postoperative follow-up at 6 months.
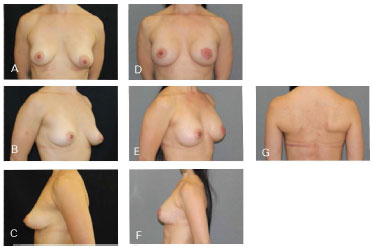
Figure 10. Clinical case 5: Periareolar mastectomy and reconstruction involving a 335 g anatomical implant, NAC graft, and symmetrization (200 g). A, B and C: Preoperative; D, E, F, and G: Postoperative follow-up at four months.
DISCUSSION
In recent decades, important developments in the detection and understanding of breast carcinoma have resulted in increasingly early diagnosis. These factors have led to increasing numbers of less aggressive mastectomies that preserve the skin and NAC, which allow ptosis and best cone shape in reconstructed breasts. Moreover, immediate mammary reconstructions have been shown to result in reduced fibrosis, retraction, and tissue atrophy. There has also been increased availability of silicone implants with high gel cohesiveness to provide breast shape stability. These associated factors have broadened the range of reconstructive options available for surgeons to correct breast defects due to cancer treatment10,16.
Within this medical landscape, we observed increased indications for local flap breast reconstruction with alloplastic materials; use of LDMF provided reduced local and systemic morbidity compared to transverse rectus abdominis muscle flap (TRAM)17,18 (Figure 11).
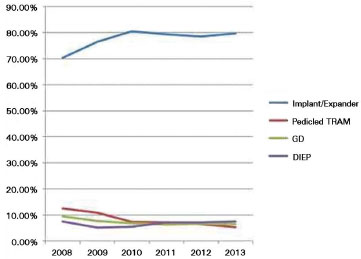
Figure 11. Number of breast reconstruction surgeries performed in the US. Source: America Society of Plastic Surgeons (www.plasticsurgery.org)
The latissimus dorsi muscle measures up to 16.3 cm wide and 29.2 cm long and the fat under the Scarpa's fascia can be mobilized with the muscle to improve implant coverage and add volume19.
We opted for an extended dissection of the latissimus dorsi associated with the fat under the Scarpa's fascia in the breast reconstruction performed in this study. This approach enabled full coverage without restricting breast implant projection, regardless of the volume used. This option reduced risks of surgical wound dehiscence of and flap necrosis caused by the mastectomy, without breast reconstruction losses. The maintenance of fat over the muscle provided a greater coverage interface between implant and skin and lower adherence of muscle to the mastectomized flap. This improved the aesthetic results and increased the tissue substrate for further breast fat grafting where necessary.
In breast cancer treatment, radiotherapy adjuvant to mastectomy is often indicated for women diagnosed with stage II and III breast cancer. Radiotherapy increases local control, disease-free survival, and overall survival20-23.
Although this cancer treatment improves survival, adjuvant radiotherapy in women with breast cancer may worsen esthetic results by inducing tissue tropism and capsular contractures and increasing the risk of breast reconstruction loss24.
In a series of 100 cases, Perdikis et al.25 observed a 6% rate of capsular contracture in patients undergoing LDMF and silicone implant. In another series of 53 cases, Venus and Prinsloo26 observed that 7.4% of patients presented with capsular contracture that required capsulotomy, while 33% of patients with capsular contracture did not require surgery. In a study published by Emory University that analyzed 83 cases, Losken et al.27 observed that radiotherapy was the only significant risk factor for LDMF-induced complications (65 vs. 35%, p = 0.05) and there was no impact on the aesthetic results.
Among patients studied in this series, we observed four cases (18.18%) of changes of implant coverage, including muscle and skin atrophy, as well as two cases (9.09%) of capsular contracture. Only one case was not associated with radiotherapy. However, due to the limited number of cases in the study sample, the relationship between adjuvant radiotherapy and late complications was not statistically significant (p = 0.635).
Therefore, although radiotherapy increases the incidence of complications in breast reconstructions, it is important for cancer treatment and plastic surgeons should find ways to address its harmful consequences.
Thus, even with potential increased risk of complications associated with radiotherapy, the psychological, social, and sexual well-being benefits suggest that immediate breast reconstruction after mastectomy should be encouraged, regardless of age and comorbidities28,29.
Seroma in the donor area of the latissimus dorsi is the most common complication. The reported rates of this complication vary from 16% to 79%16,27,30-36. However, the significance of seroma as a major complication requiring surgical intervention is low35,37.
Seroma occurred 44% of cases in this study. This relatively high rate is probably due to the extensive detachment of the latissimus dorsi and lack of adhesion points in the dorsal region. All cases were treated effectively with outpatient percutaneous puncture. If we consider seroma a predictable complication with easy resolution, its high incidence should not be overemphasized at the expense of performing a procedure that provides safe and satisfactory aesthetic results.
Among early complications, Gart et al.17 reported 5.7% reoperations, 3.3% skin infections, 1.3% flap necrosis, and 0.6% surgical wound dehiscence as well as 3.2% clinical complications in a study of 1,079 patients in the American College of Surgeons National Surgical Improvement Program (ACS-NSQIP) database who underwent LDMF.
The early complications detected in this case series included three cases each (14%) partial necrosis of the mastectomized skin and partial surgical wound dehiscence. These events probably occurred due to local cancer treatment, which resulted in thin and hypoperfused mastectomized flaps. No latissimus dorsi flap necrosis, infections, clinical complications, or reoperations were observed in these cases.
Even with the recent focus in scientific literature and the surgical practice on microsurgical flaps, pedicled LDMF remains an option for a large number of breast reconstruction procedures and should not be considered secondary choice for flap procedures17.
In addition to being a large flap with known and reliable skin territory that typically presents fewer complications compared to other autologous flaps, LDMF offers additional advantages, including fast recovery, high patient satisfaction, and minimal morbidity of the donor area30,38-40.
CONCLUSION
LDMF associated with silicone implants is a safe and reliable option for immediate breast reconstructions after mastectomies.
REFERENCES
1. Brasil. Ministério da Saúde. Estimativa 2014: incidência do câncer de mama no Brasil. Instituto Nacional José de Alencar Gomes da Silva [Acesso 19 de maio de 2014]. Disponível em: http://www.inca.gov.br
2. Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894;20(5):497-555. DOI: http://dx.doi.org/10.1097/00000658-189407000-00075
3. Patey DH, Dyson WH. The prognosis of carcinoma of the breast in relation to the type of operation performed. Br J Cancer. 1948;2(1):7-13. DOI:http://dx.doi.org/10.1038/bjc.1948.2
4. Madden JL. Modified radical mastectomy. Surg Gynecol Obstet. 1965;121(6):1221-30. PMID: 5851617
5. Fisher B, Bauer M, Margolese R, Poisson R, Pilch Y, Redmond C, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665-73. PMID: 3883167 DOI:http://dx.doi.org/10.1056/NEJM198503143121101
6. Veronesi U, Saccozzi R, Del Vecchio M, Banfi A, Clemente C, De Lena M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305(1):6-11. PMID: 7015141 DOI:http://dx.doi.org/10.1056/NEJM198107023050102
7. Lambert PA, Kolm P, Perry RR. Parameters that predict nipple involvement in breast cancer. J Am Coll Surg. 2000;191(4):354-9. PMID: 11030239
8. Laronga C, Kemp B, Johnston D, Robb GL, Singletary SE. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol. 1999;6(6):609-13. DOI: http://dx.doi.org/10.1007/s10434-999-0609-z
9. Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87(6):1048-53. PMID: 1852020 DOI: http://dx.doi.org/10.1097/00006534-199106000-00006
10. Claro Jr. F, Costa DV, Pinheiro AS, Pinto-Neto AM. Complicações em reconstrução mamária total em pacientes mastectomizadas por câncer de mama: análise comparativa de longo prazo quanto a influência da técnica, tempo de cirurgia, momento da reconstrução e tratamento adjuvante. Rev Bras Cir Plást. 2013;28(1):85-91. DOI: http://dx.doi.org/10.1590/S1983-51752013000100015
11. Tansini I. Sopra il mio nuovo processor di amputazione della mammella. Gazz Mal Ital. 1906;57:141.
12. Olivari N. The Latissimus flap. Br J Plast Surg. 1976(2);29:126-8. PMID: 776304
13. Schneider WJ, Hill HL Jr, Brown RG. Latissimus dorsi myocutaneous flap for breast reconstruction. Br J Plast Surg. 1977;30(4):277-81.
14. Bostwick J 3rd, Vasconez LO, Jurkiewicz MJ. Breast reconstruction after a radical mastectomy. Plast Reconstr Surg. 1978;61(5):682-93. PMID:347475 DOI: http://dx.doi.org/10.1097/00006534-19780500-000004
15. Hammond DC. Latissimus dorsi flap breast reconstruction. Plast Reconstr Surg. 2009;124(4):1055-63. PMID: 19935289 DOI:http://dx.doi.org/10.1097/PRS.0b013e3181b6bf05
16. Di Lamartine J, Galdino Júnior J, Daher JC, Guimarães GS, Camara Filho JPP, Borgatto MS, et al. Reconstrução mamária com retalho do músculo grande dorsal e materiais aloplásticos: análise de resultados e proposta de nova tática para cobertura do implante. Rev Bras Cir Plást. 2012;27(1):58-66. DOI: http://dx.doi.org/10.1590/S1983-51752012000100010
17. Gart MS, Smetona JT, Hanwright PJ, Fine NA, Bethke KP, Khan SA, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-38. PMID: 23211118
18. Cammarota MC. Reconstrução de mama com retalho de grande dorsal: estudo das pacientes operadas no período de junho de 2003 a junho de 2005 [Tese para concurso de titular]. In: XLIII Congresso Brasileiro de Cirurgia Plástica; 2006 Nov 11-14; Recife, Brasil.
19. Hazan ASB, Nahas FX, Barbosa MVJ, Pineda E, Juliano Y, Ferreira LM. Análise anátomo-histológica das subunidades musculares do músculo grande dorsal. Rev Soc Bras Cir Plást. 2006;21(4):203-10.
20. Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337(14):949-55. DOI:http://dx.doi.org/10.1056/NEJM199710023371401
21. Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641-8. PMID:10335782
22. Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337(14):956-62. PMID: 9309100 DOI: http://dx.doi.org/10.1056/NEJM199710023371402
23. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al.; Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087-106.
24. Shah C, Kundu N, Arthur D, Vicini F. Radiation therapy following postmastectomy reconstruction: a systematic review. Ann Surg Oncol. 2013;20(4):1313-22. DOI: http://dx.doi.org/10.1245/s10434-012-2689-4
25. Perdikis G, Koonce S, Collis G, Eck D. Latissimus dorsi myocutaneous flap for breast reconstruction: bad rap or good flap? Eplasty. 2011;11:e39. PMID: 22031843
26. Venus MR, Prinsloo DJ. Immediate breast reconstruction with latissimus dorsi flap and implant: audit of outcomes and patient satisfaction survey. J Plast Reconstr Aesthet Surg. 2010;63(1):101-5. DOI: http://dx.doi.org/10.1016/j.bjps.2008.08.064
27. Losken A, Nicholas CS, Pineel XA, Carlson GW. Outcomes evaluation following bilateral breast reconstruction using latissimus dorsi myocutaneous flaps. Ann Plast Surg. 2010;65(1):17-22. DOI: http://dx.doi.org/10.1097/SAP.0b013e3181bda349
28. Atisha D, Alderman AK, Lowery JC, Kuhn LE, Davis J, Wilkins EG. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg. 2008;247(6):1019-28. PMID:18520230 DOI: http://dx.doi.org/10.1097/SLA.0b013e3181728a5c
29. Veronesi P, Ballardini B, De Lorenzi F, Magnoni F, Lissidini G, Caldarella P, et al. Immediate breast reconstruction after mastectomy. Breast. 2011;20 Suppl 3:S104-7. DOI: http://dx.doi.org/10.1016/S0960-9776(11)70305-8
30. Delay E, Gounot N, Bouillot A, Zlatoff P, Rivoire M. Autologous latissimus breast reconstruction: a 3-year clinical experience with 100 patients. Plast Reconstr Surg. 1998;102(5):1461-78. PMID: 9774000 DOI: http://dx.doi.org/10.1097/00006534-199810000-00020
31. Bonomi S, Settembrini F, Salval A, Gregorelli C, Musumarra G, Rapisarda V. Current indications for and comparative analysis of three different types of latissimus dorsi flaps. Aesth Surg J. 2012;32(3):294-302. DOI: http://dx.doi.org/10.1177/1090820X12437783
32. Chang DW, Youssef A, Cha S, Reece OR. Autologous breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg. 2002;110(3):751-9.
33. Munhoz AM, Montag E, Fels KW, Arruda EG, Sturtz GP, Aldrighi C, et al. Outcome analysis of breast-conservation surgery and immediate latissimus dorsi flap reconstruction in patients with T1 to T2 breast cancer. Plast Reconstr Surg. 2005;116(3):741-52. PMID: 16141810 DOI:http://dx.doi.org/10.1097/01.prs.0000176251.15140.36
34. Roy MK, Shrotia S, Holcombe C, Webster DJ, Hughes LE, Mansel RE. Complications of latissimus dorsi myocutaneous flap breast reconstruction. Eur J Surg Oncol. 1998;24(3):162-5. PMID: 9630851 DOI: http://dx.doi.org/10.1016/S0748-7983(98)92810-4
35. Sternberg EG, Perdikis G, McLaughlin SA, Terkonda SP, Waldorf JC. Latissimus dorsi flap remains an excellent choice for breast reconstruction. Ann Plast Surg. 2006;56(1):31-5. PMID: 16374092 DOI: http://dx.doi.org/10.1097/01.sap.0000186463.07617.6f
36. Rios JL, Pollock T, Adams WP Jr. Progressive tension sutures to prevent seroma formation after latissimus dorsi harvest. Plast Reconstr Surg. 2003;112(7):1779-83. PMID: 14663220 DOI: http://dx.doi.org/10.1097/01.PRS.0000090542.68560.69
37. Bailey SH, Oni G, Guevara R, Wong C, Saint-Cyr M. Latissimus dorsi donor-site morbidity: the combination of quilting and fibrin sealant reduce length of drain placement and seroma rate. Ann Plast Surg. 2012;68(6):555-8 PMID: 21629082 DOI: http://dx.doi.org/10.1097/SAP.0b013e318216b65c
38. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg. 1992(3);74:377-82. PMID: 1548264 DOI: http://dx.doi.org/10.1097/00005131-199212000-00068
39. Russell RC, Pribaz J, Zook EG, Leighton WD, Eriksson E, Smith CJ. Functional evaluation of latissimus dorsi donor site. Plast Reconstr Surg. 1986;78(3):336-44. DOI: http://dx.doi.org/10.1097/00006534-198609000-00009
40. Dutra AK, Neto MS, Garcia EB, Veiga DF, Netto MM, Curado JH, et al. Patients' satisfaction with immediate breast reconstruction with a latissimus dorsi musculocutaneous flap. J Plast Surg Hand Surg. 2012;46(5):349-53. DOI: http://dx.doi.org/10.3109/2000656X.2012.704726
1. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
2. Instituto Brasileira de Controle do Câncer, São Paulo, SP, Brazil
3. Sociedade Brasileira de Mastologia, Rio de Janeiro, RJ, Brazil
Institution: Instituto Brasileiro de Controle do Câncer, São Paulo, SP, Brazil.
Corresponding author:
Gabriel Salum D'Alessandro
Rua Oscar Freire, 2250, conjunto 408, Pinheiros
São Paulo, SP, Brazil Zip Code 05409-011
E-mail: dr.gabriel.dalessandro@gmail.com
Article received: December 5, 2014.
Article accepted: March 15, 2015.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter