

Original Article - Year 2015 - Volume 30 -
The formation of the latissimus dorsi muscle flap in the dorsal decubitus position
A sistematização do retalho do músculo latíssimo do dorso em decúbito dorsal
ABSTRACT
INTRODUCTION: The latissimus dorsi muscle is located on the lateral-posterior chest wall. The latissimus dorsi muscle flap (LDMF) has extensive applications due to its consistent anatomy, which contributes to its use in plastic surgery. This muscle can be dissected in the dorsal decubitus (DD) position; this removes the need for a change in patient position and enables microsurgical reconstruction to be performed. This study aimed to describe the technique used for the dissection and transfer of the LDMF in the DD position.
METHODS: We evaluated 10 cases of LDMF formation performed in the University Hospital of the Federal University of Alagoas. The surgical procedures of individualization of the flaps and their positioning in the receiver region were similar in all ten cases. The evaluation of the muscle and the demarcation of the flap were performed with the patient in the standing position. Then, with the patient in the DD position, anesthetic induction was initiated and the operating field prepared. The neurovascular pedicle was formed by incising the top edge of the ellipse that delimits the LDMF, dissecting the insertion tendon of the muscle, and removing the flap necessary for the reconstruction of the receiver area.
RESULTS: The patient was in the DD position during the intraoperative period, even during the dissection of muscle flaps, and the technique used had no surgical disadvantages.
CONCLUSION: It is possible to perform the dissection and individualization of the LDMF with the patient in the DD position.
Keywords: Dorsal decubitus position; Superficial muscles of the back; Surgical flaps.
RESUMO
INTRODUÇÃO: O músculo latíssimo do dorso localiza-se na parede latero-posterior do tórax. O retalho do músculo latíssimo do dorso (RMLD) tem uma extensa aplicabilidade devido a sua anatomia pouco variável contribuindo, assim, para sua utilização em Cirurgia Plástica. A possibilidade de dissecar este músculo em decúbito dorsal (DD) dispensa a mudança de posição do paciente e cria uma opção para reconstruções microcirúrgicas. Este estudo objetiva sistematizar a técnica utilizada para dissecção do RMLD em DD.
MÉTODO: Foram avaliados 10 casos de RMLD realizados no Hospital Universitário da Universidade Federal de Alagoas, os procedimentos cirúrgicos de individualização dos retalhos e seu posicionamento na região receptora foram semelhantes nos dez casos. É realizada a avaliação do músculo e a demarcação do retalho com o paciente em pé. Depois, com o paciente em DD, faz-se a indução anestésica e a preparação do campo a ser operado. Faz a abordagem do pedículo vásculo-nervoso por incisão na margem superior da elipse que delimita o RMLD, secciona o tendão de inserção do músculo e retira-se o retalho necessário à área receptora a ser reconstruída.
RESULTADOS: A posição do paciente no intraoperatório foi o DD, inclusive durante a dissecação dos retalhos musculares, e a técnica utilizada não proporcionou desvantagens do ato cirúrgico.
CONCLUSÃO: É possível realizar a dissecção e individualização do RMLD estando o paciente em DD.
Palavras-chave: Decúbito dorsal; Músculos superficiais do dorso; Retalhos cirúrgicos.
The latissimus dorsi muscle (LDM) is one of the most important elements of the lateral-posterior chest wall. The latissimus dorsi muscle flap (LDMF) has been used extensively in surgical reconstruction techniques and its implementation has evolved in recent decades. However, the use of this flap is not recent1. The LDMF was first used in 1896 by Tansini2 to reconstruct the thoracic region after mastectomy. However, its wide acceptance in Europe only occurred at the beginning of the 20th century, with the dissemination of important work in 1912 by D'Este3. From 1939, the application of the LDMF was cited for various purposes in plastic surgery.
The LDMF has great versatility, and can be used in breast reconstruction4-6, the coverage of defects in the thoracic wall7, head and neck reconstructions1,8, the functional restoration of the shoulder and arm9,10, the closure of myelomeningocele11, and in the reconstruction of the abdominal wall, lower extremities, and cranial vertex1,8. In addition to the extensive applicability of this flap, its consistent anatomy contributes to its frequent use in plastic surgery8.
The position of the patient during the intraoperative period is of extreme importance to the individualization and rotation of the LDMF. If the patient is positioned in the ventral or lateral decubitus position for the dissection of the LDM, he/she will have to be repositioned during the transfer of the flap; this may increase morbidity and surgical time8. Dissecting the LDM with the patient in the dorsal decubitus (DD) position eliminates the need for repositioning the patient during surgery and provides the option of using microsurgical reconstruction8.
OBJECTIVE
This study aimed to describe the feasibility of the technique described by Jean-Marie Servant and Marc Revol for the dissection and transfer of the LDMF while in the DD position in plastic surgery reconstructions.
METHOD
This study followed the standards of the National Council for Ethics in Research (CEP/CONEP), resolution 96/166. CAAE approval number: 41655214.4.0000.5013.
Ten cases of LDMF formation carried out at the University Hospital of the Federal University of Alagoas (HU/UFAL) were evaluated8. All patients were registered using computerized and standardized records. Seventy percent of the patients were male and thirty percent female, with an age range of 18 to 63 years (mean 41.7 years). The pre-operative and surgical procedures for the formation of the LDMF and its positioning in the receiving area bed were similar in all cases.
The pre-operative period in these surgeries requires the knowledge of pre-exposure to radiation and/or surgical interventions, whether in the donor area or at the receiving area. In children, radiographic examination of the spine is recommended; this can be used as a basis of comparison for future assessments. Complementary examinations such as arteriography and Doppler were not requested, as they were not considered necessary.
A dynamic assessment of the LDM is performed with the patient standing, with the arm in forced adduction, and with the hands positioned on the iliac crests. The demarcation of the medial limit is performed with the muscle contracted, so that it is possible to see and palpate the medial margin of this muscle and the axis of rotation of the flap corresponding to the origin of the vascular pedicle, situated in the center of the axillary fossa, and with the arm in elevation and in complete abduction.
The intraoperative procedure for the rotation of the LDMF is as follows: the anesthetic induction begins after the flap has been demarcated. General inhalation anesthesia with tracheal intubation was chosen. The patient is in the DD position with the arm in abduction on an auxiliary support of a hand operating table. A pad (a large surgical drape wrapped about itself, of approximately 30 cm in length and 14 cm in diameter) is placed longitudinally along the paravertebral line of the side to be operated on, thus elevating the lateral portion of the patient.
The neurovascular pedicle is approached first after the demarcation of the flap, through an inverted "V" incision on the top edge of the ellipse that defines the LDM flap with a medial apex and approximately 140º of angulation, involving the skin and subcutaneous tissue. Then, the incision is extended according to the constitution and design of the desired flap. For a muscle flap, the extension is longitudinal, following the midaxillary line. For a fasciocutaneous muscle flap, the extension meets the medial demarcation, observing the relations of the LDM with the muscles of the thorax and abdomen.
The incision of the lateral skin segment is guided by the width required to cover the skin defect and the distance to the proximal end, with the aim of facilitating the approach of the pedicle region. It is important that the width of the skin flap is not greater than 10 cm.
In the dissection of the LDM, anatomical knowledge is imperative. The subscapular artery, vein, and nerve arise from the area of the axillary artery and vein. The subscapular artery and vein descend the chest wall, branching to form the thoracodorsal artery and vein and the circumflex artery and vein of the scapula. In the dissection of the LDM, visual identification of the subscapular and circumflex arteries of the scapula are not needed, but the thoracodorsal artery will usually be viewed. The identification of the insertion tendon of the LDM and its section is of great importance, and is crucial for the proper rotation of the flap. The authors always identify the tendon and section it above the thoracodorsal vessels when they carry out rotation of the LDM. After sectioning the insertion tendon of the LDM, the flap can be positioned on the receiving area to carry out the reconstruction.
RESULTS
In the present study, the patient was in the DD position during all surgical procedures, providing benefits for the patient and the surgical team (Figures 1 to 4).
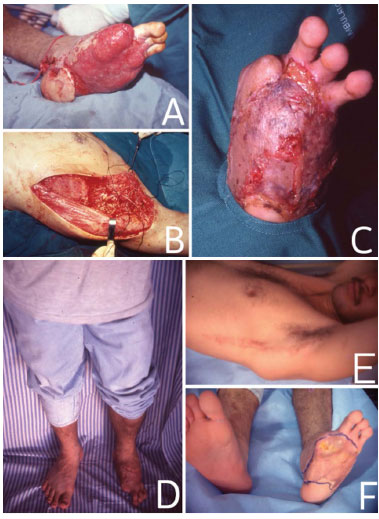
Figure 1. In (A), traumatic lesion in the left foot. In (B), surgery for the formation of LDMF in the DD position. In (C), skin grafts performed 4 weeks after the placement of the microsurgical muscle flap. In (D), The patient in the standing position, 1 year after the surgery. In (E), the surgical scar 1 year after the surgery. In F, the receiving area 1 year after the surgery.
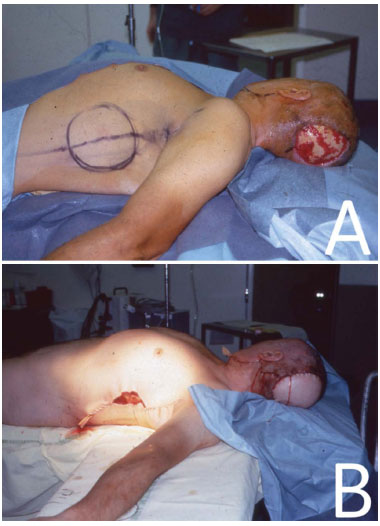
Figure 2. In (A), patient in the DD position with the demarcated LDMF. In (B), immediate postoperative positioning with the LDMF for the reconstruction of the lesion area in the occipital region that was caused by a tumor.
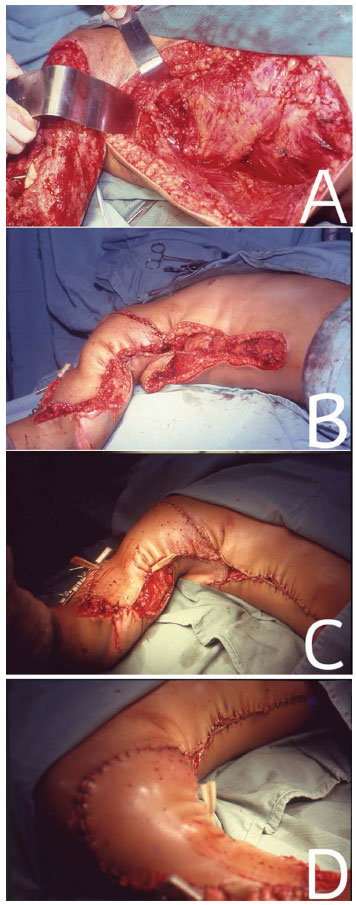
Figure 3. In (A), approach of the LDM with the patient in the DD position for correction of a traumatic lesion in the right upper limb. In (B), LDMF positioned on traumatic injury. In (C) and (D), immediate post-operative period.
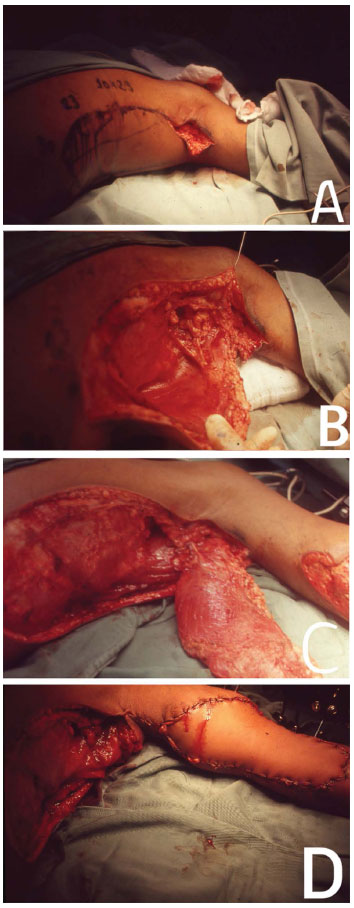
Figure 4. In (A), the beginning of the approach to dissect and transfer the LDMF with the patient in the DD position. In (B), intraoperative. In (C), the mobilized LDM. In (D), coverage of the traumatic injury in the right upper limb.
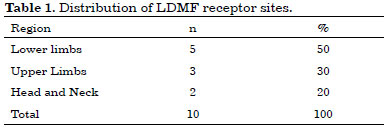
Note that in these cases, 50% of the receiving areas were on the lower limbs (LLs), 30% on the upper limbs (ULs), and 20% in the region of the head and/or neck (Table 1). Regarding the cause of injuries, 50% were because of trauma and the other 50% because of tumor lesions (Table 2).

DISCUSSION
In this study, all the LDMFs were dissected with the patient in the DD position, as described by Jean Marie Servant8. This was important for establishing whether the use of the DD position was feasible during the surgical procedure and for determining whether it brought greater benefits than the more common positions, such as the lateral and ventral decubitus positions.
The use of the DD position in LDMF operations enables the possibility of concurrent action by two teams, one working in the flap donor area and the other in the receiving area. This can help in decreasing the intraoperative time. In cases of microsurgical flaps, the surgical procedure becomes simpler. The surgeon is more comfortable as the dissection and hemostasis can be performed while seated. There are no changes in the patient position; the donor area preparation becomes easier, and can even be performed by another team, while the surgeon concomitantly may start flap anastomosis. The approach of the pedicle, which is short when it penetrates the LDM, as it is near the anterior margin of the muscle, is facilitated by the DD position and by having the arm placed naturally in abduction8. The patient is anesthetized in the DD position, and therefore the anesthesia team can work with the patient in a fixed position, without having to change the patient position or the surgical field; this facilitates the mounting of the endotracheal tube and the monitoring of the clinical parameters.
It is believed that the low failure rate of LDMFs is due to the flap characteristics, such as a long large-caliber vascular pedicle, as well as its consistent anatomy and the limited presence of atheromatous plaques in the vascular lumen of the subscapular and thoracodorsal arteries8.
One should note that there is no comparison in the literature for the degree of surgical difficulty with the patient in the DD position, but in this position, LDMF individualization is feasible. There were no complications that could be attributed solely to the surgical position.
CONCLUSION
It is possible to perform the dissection and individualization of LDMF with the patient in the DD position.
REFERENCES
1. Bartlett SP, May JW Jr, Yaremchuk MJ. The latissimus dorsi muscle: a fresh cadaver study of the primary neurovascular pedicle. Plast Reconstr Surg. 1981;67(5):631-6.
2. Tansini I. Sopra il mio nuovo processo di amputazione della mammella. Gaz Med Ital. 1906;57:141.
3. D'Este S. La technique de l'amputation de la mamelle pour carcinoma mammaire. Rev Chir (Paris). 1912;45:164-210.
4. Olivari N. The latissimus flap. Br J Plast Surg. 1976;29(2):126-8.
5. Muhlbauer W, Olbrisch R. The latissimus dorsi myocutaneous flap for breast reconstruction. Chir Plast (Berlin) 1977;4:27-34.
6. Bostwick J 3rd, Vasconez LO, Jurkiewicz MJ. Breast reconstruction after a radical mastectomy. Plast Reconstr Surg. 1978;61(5):682-93.
7. Davis HH, Tollman JP, Brush JH. Huge chondrosarcoma of rib, report of a case. Surgery. 1949;26(4):699-704.
8. Andrade FAG, Servant JM, Perreira LM, Revo M, Traber H, Nascimento Júnior CP, et al. Decúbito dorsal no retalho microcirúrgico do músculo grande dorsal - Técnica de J. M. Servant. Rev Soc Bras Cir Plást. 2000;15(1):35-46.
9. Schottstaedt ER, Larsen LJ, Bost FC. Complete muscle transposition. J Bone Joint Surg Am. 1955;37-A(5):897-918.
10. Zancolli E, Mitre H. Latissimus dorsi transfer to restore elbow flexion. An appraisal of eight cases. J Bone Joint Surg Am. 1973;55(6):1265-75.
11. Desprez JD, Kiehn CL, Eckstein W. Closure of large meningomyelocele defects by composite skin-muscle flaps. Plast Reconstr Surg. 1971;47(3):234-8.
1. Universidade Federal de Alagoas, Maceió, AL, Brazil
2. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
Institution: Hospital Universitário da Universidade Federal de Alagoas (HU/UFAL), Maceió, AL, Brazil.
Corresponding author:
Fernando Antônio Gomes de Andrade
Rua Osvaldo Sarmento, 63, Farol
Macéio, AL, Brazil Zip code 57051510
E-mail: fernandogomescirurgiaplastica@hotmail.com
Article received: June 22, 2014.
Article accepted: April 21, 2015.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter