

Original Article - Year 2015 - Volume 30 -
Protocol for bilateral application of botulinum toxin type A to avoid asymmetry during treatment of hemifacial spasms
Protocolo de aplicação bilateral de toxina botulínica tipo A para evitar assimetria no tratamento de espasmo hemifacial
ABSTRACT
INTRODUCTION: Hemifacial spasm (HFS) is characterized by the involuntary tonic-clonic movement of the muscles of the hemiface. It is usually treated with botulinum toxin (BTX). The classically described unilateral application of BTX results in an asymmetry similar to facial paralysis. The aim of this study was to standardize the treatment of HFS by applying BTX bilaterally to prevent the occurrence of iatrogenic facial asymmetry.
METHODS: The outcomes of 66 applications in 15 patients were analyzed according to the protocol of the facial paralysis service, to which pretarsal sites were added on the HFS side. On reassessment 15 days later, a complementary dose was administered to patients who exhibited some residual degree of spasm or asymmetry with the aim of determining the dose required to achieve satisfactory spasm control without causing facial asymmetry.
RESULTS: The total mean dose was 20.2 U at the contralateral side and 28.4 U at the spasm side (a total dose of 48.6 U per application). There was a significant difference between the doses applied to the zygomaticus, orbicularis oris, and orbicular oculi muscles on each hemiface.
CONCLUSIONS: The proposed bilateral BTX application technique was effective in controlling HFS and prevented iatrogenic asymmetry. In general, application should be performed at a ratio of 1:1.5 U in the orbicularis oculi (lateral portion) and 1:2 U in the orbicularis oris. In the remaining muscles, the same dose should be administered on both sides and an additional dose can be applied 15 days later if some degree of spasm is present. The pretarsal region of the orbicularis oculi muscle is the only area for which BTX application on the healthy side is unnecessary.
Keywords: Botulinum toxin type A; Facial paralysis; Facial asymmetry/therapy; Hemifacial spasm
RESUMO
INTRODUÇÃO: O espasmo hemifacial (EHF) caracteriza-se por movimento tônico-clônico involuntário da musculatura de uma hemiface. O tratamento tem sido realizado com aplicação de toxina botulínica (TxB). A aplicação unilateral classicamente descrita resulta em assimetria semelhante à paralisia facial. O objetivo desse trabalho foi normatizar o tratamento do EHF bilateralmente com TxB, a fim de prevenir a ocorrência de assimetria facial iatrogênica.
MÉTODO: Foram analisadas 66 aplicações em 15 pacientes, seguindo o protocolo do serviço para paralisia facial, acrescentado de pontos pré-tarsais no lado com EHF. Foi feita dose complementar na reavaliação após 15 dias nos pacientes que apresentavam algum grau residual de espasmo ou assimetria, buscando-se a dose necessária para alcançar controle satisfatório do espasmo sem causar assimetria facial.
RESULTADOS: A dose média total foi 20,2 U do lado não acometido e 28,4 U do lado acometido, totalizando 48,6 U por aplicação. Houve diferença significante entre as hemifaces na dose para os músculos zigomático, orbicular da boca e orbicular dos olhos.
CONCLUSÕES: A técnica proposta de aplicação bilateral de TxB controlou adequadamente o EHF e evitou assimetria iatrogênica. Como regra geral, a aplicação deve ser feita na proporção de 1:1,5 U no orbicular dos olhos (porção lateral) e 1:2 U no orbicular da boca. Nos demais músculos, a dose nos dois lados deve ser a mesma, realizando-se dose de reforço em 15 dias caso permaneça algum grau de espasmo. O único local com pontos exclusivos do lado acometido é a região pré-tarsal do músculo orbicular do olho.
Palavras-chave: Toxina botulínica Tipo A; Paralisia facial; Assimetria facial/terapia; Espasmo hemifacial.
Hemifacial spasm (HFS) is characterized by an involuntary tonic-clonic movement of the muscles in the hemiface. It affects the facial expression muscles innervated by the ipsilateral facial nerve. HFS can be divided into two types: primary and secondary. The etiology of the former has not been fully determined. The latter can be caused by trauma, infection, or post-paralytic facial syndrome1,2.
The treatment of HFS with oral drugs (for example, carbamazepine, gabapentin) has not resulted in satisfactory long-term outcomes, nor has extracranial surgery (rhizotomy, myotomy, neural anastomosis) or intracranial surgery such as microvascular decompression of the facial nerve1. Currently, the first therapeutic choice is the direct application of botulinum toxin type A to the facial muscles3,4.
Since Elston5 treated HFS with botulinum toxin for the first time in1985, the effectiveness of this treatment has been demonstrated consistently. Several studies reported the successful treatment of 76-100% of patients6-8.
Beyond this point, the literature is controversial. Some studies were predominantly based on cases of blepharospasm and asymmetry of the upper third of the face, in which the unilateral application of toxin was the standard method9-12. However, when unilateral application was also used in the lower third, it exacerbated muscle weakness in the treated hemiface and caused asymmetry of the facial expression. In 1991, Borodic et al.13 initiated studies in which doses of toxin were applied to both the upper third and two lower thirds of the face to soften facial asymmetry. The author noted a common complication - iatrogenic unilateral facial asymmetry - when the toxin was applied only on one hemiface. This complication was mostly observed when treating the middle and lower thirds of the face.
This asymmetry gives patients an appearance similar to that of facial paralysis, with the smile slanting to the contralateral side (not treated with botulinum toxin), which causes great social discomfort. At this point in the evolution of HFS treatment using botulinum toxin type A,5,10,14-19 the discussion has moved to the standardization of sites for the bilateral application of botulinum toxin since the first application9,18,20. The spasm would thus be controlled by the application of the toxin to the spasm side and iatrogenic asymmetry would be avoided by applying the toxin to the contralateral side.
OBJECTIVE
The objective of this study was to standardize the treatment of HFS with the bilateral application of botulinum toxin type A (from the first application) to standardized muscle sites of the face to prevent the occurrence of iatrogenic facial asymmetry.
METHODS
The present study was prospective from the start and was conducted between May 2005 and June 2012 (the end of data collection). The study was approved by the Ethics Committee of our institution (nº 393/09). Patients were treated by the Plastic and Aesthetics Surgery Group of the Faculty of Medicine, University of São Paulo. The PubMed database of the US National Library of Medicine of the National Institutes of Health was searched for relevant literature using the following keywords: botulinum toxin type A, facial paralysis, facial asymmetry/therapy, and facial spasm.
Sixty-six applications of botulinum toxin in 15 patients were analyzed. The treatments were administered with 5- to 7-month intervals between applications. All patients gave their written informed consent. All patients were interviewed and their HFS etiologies were investigated. The mean patient age was 63.2 years. The cohort was 78.5% female and 21.5% male. The left side was affected in 60% of cases.
All study patients were administered botulinum toxin type A (Botox®; Allergan, Inc., Irvine, California, USA). The sites of application were determined according to the protocol of the facial paralysis service, standardized by Salles21 and Salles et al.22, 0.5 or 1 U were applied to the pretarsal sites on the HFS side to control spasms of the orbicular oculi (Figure 1).
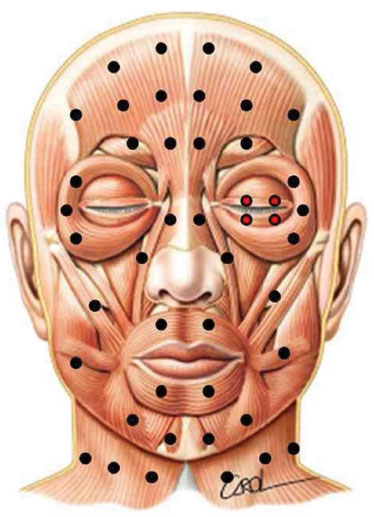
Figure 1. Standardized points of application of botulinum toxin for the treatment of hemifacial spasm to avoid iatrogenic asymmetry. Pretarsal sites were added to the original protocol, seen in red.
The toxin was applied to all muscles in which spasms were observed at a dose of 1-2 U. Symmetrical sites were marked on the contralateral side, in the muscles that cause asymmetry in the upper, middle, and lower thirds of the face.
The muscles analyzed for toxin application were as follows: frontal (2-5 sites), corrugator/procerus (1-3 sites), orbicularis oculi (lateral portion) (2-3 sites), pretarsal (2-4 sites), nasalis (1 site), levator labii superioris (1 site), zygomaticus (1-3 sites), risorius (1-2 sites), depressor labii inferioris (1-3 sites), mentalis (1-2 sites), and orbicularis oris (1-2 sites). The platysma muscle was excluded from this analysis.
All patients were evaluated objectively using standardized photos of the front view, side view, and three-fourth view with a resting face and smiling face, while contracting the corrugator and nasalis muscles, and while protruding the lip and depressing the lower lip. Patients were also filmed both statically and while moving their facial expression muscles. They were also evaluated subjectively at the start of the treatment and after each application, and the degrees of satisfaction and symmetry were always assessed by the same professional. Patients were followed up 15 days after toxin application, and at each visit, patients were reassessed by the first author and photographed to compare the treatment effects over time.
A complementary dose was administered 15 days after the application of botulinum toxin type A only in patients who exhibited a residual degree of HFS or static or dynamic asymmetry. These patients were reassessed after another 15 days to determine the results and side effects. The total dose was defined as the dose needed to achieve satisfactory spasm control without causing facial asymmetry.
RESULTS
During the 6-year study period, the mean number of applications was 4.7 per patient (varying between 4 and 16 applications). The interval between applications was 5 to 7 months.
The cause of spasm was idiopathic in 13 cases (86.6%), trauma in one case, and stroke in one case.
The mean doses (UI) of botulinum toxin type A used per application and per muscle group in each side of the face are listed in Table 1.
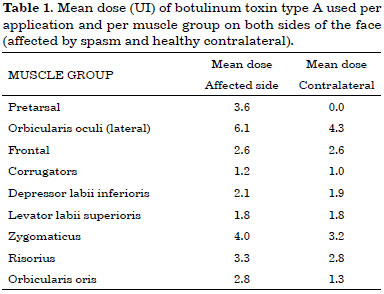
The total mean dose of botulinum toxin per application was 20.2 U at the contralateral side and 28.4 U at the spasm side, for a total mean dose of 48.6 U per application considering the whole face. Figure 2 shows an example of the outcome of toxin application (static and dynamic); pretarsal sites are seen on the side with HFS.
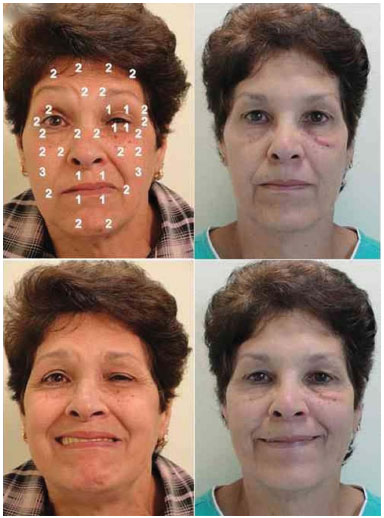
Figure 2. A 58-year-old patient with hemifacial spasm on the left side for 9 years before and after the application of botulinum toxin. The sites of application and dose used at each site are shown for the resting face (upper photographs) and smiling face (lower photographs). Note that after application, the resting face shows relaxation of the orbicularis oculi and softening of the left nasolabial fold, in addition to improved smile quality after treatment.
The following complications were observed: slight speech disturbance (50%), slight difficulty in swallowing liquids (35.7%), dry eyes (14.2%), and mild ectropion (6%). These lasted for a maximum of 18 days. All patients were satisfied with the treatment, both with regard to spasm control and facial symmetry.
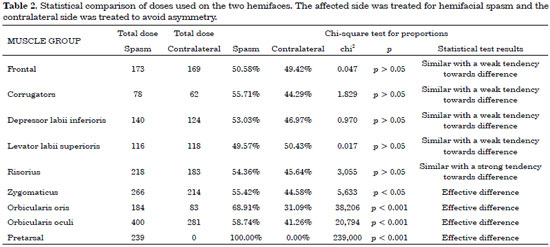
Table 2 shows a statistical comparison of the two sides of the face. The muscle groups with a p-value > 0.05 were not statistically different, and therefore the dose was the same on both sides of the face.
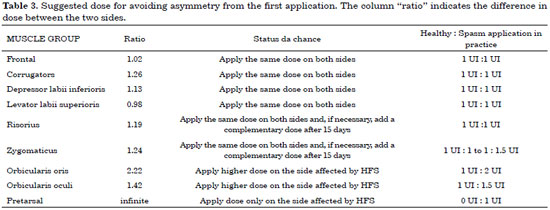
Table 3 shows the proposed protocol, based on a statistical analysis of the doses needed to treat the spasm without causing iatrogenic asymmetry in this sample.
DISCUSSION
There is consensus in the literature regarding the efficacy of botulinum toxin for the treatment of various disorders5,9-12,14-19,21-27. The toxin is endocytosed by the presynaptic neuron and inhibits the release of acetylcholine at the neuromuscular junction, which leads to temporary complete or incomplete paralysis of the muscles in the injected area16,28.
Since Elston5 treated HFS with botulinum toxin type A for the first time in 1985, studies have reported facial spasm control results of 76-100%6-8.
However, currently available studies on the method of application vary considerably with regard to sample size, ranging from 6 cases13 to 332 cases9. Moreover, most studies only consider the upper third of the face.
In addition, unilateral toxin application is used by most authors 9-11,14,27, with the exception of Borodic et al.13 and Colakoglu et al.29, both of whom studied a small number of patients. There is no standardized protocol for the bilateral application of botulinum toxin in patients with HFS in the literature. This study is therefore unique in that it analyzed 66 bilateral applications, and included muscles of the upper, middle, and lower thirds of the face.
Complications described in the literature include paralysis of the orbicularis oris with difficulty in oral continence (38.8% of cases)25, dry eyes (19.8%)10, eyelid ptosis (between 10.9% and 52.7%)9,10,14,24,27, and diplopia (between 2% and 27.7%)9,10,12,14,27. Complications in the present study were similar to those described above and occurred in similar proportions: slight speech disturbance (50%), slight difficulty in swallowing liquids (35.7%), dry eyes (14.2%), and mild ectropion (6%). The maximum duration was 18 days.
The average annual cost of medication for the treatment of HFS was 804.13 R$ per patient (8 R$ per unit), which is less expensive and more effective than the surgical procedures previously performed for this condition, including rhizotomy, myotomy, and decompression of the facial nerve.
The mean dose required to completely control HFS decreased over time. The intervals between applications showed an increasing trend. These subjective findings require further confirmation by larger studies.
The present study showed that the pretarsal region should only be treated on the side affected by spasm. There was a statistical difference between the doses required to control spasm without causing iatrogenic asymmetry in 3 muscle groups: the dose administered to the zygomaticus on the affected side was 1.24 times higher than that administered to the contralateral side, the dose administered to the orbicularis oris muscles was 2.22 times higher, and the dose applied to the orbicularis oculi muscles was 1.42 times higher.
There was also a statistical difference with regard to the remaining analyzed muscle groups (frontal, corrugator, depressor labii inferioris, levator labii superioris, and risorius). These exhibited dose differences as well, with the spasm side requiring higher doses of toxin; however, the differences were not statistically significant (p > 0.05).
The physician should start the injection by determining the dose needed in each muscle with dystonia on the spasm side, using doses of 0.5 to 2 U. On the contralateral side, the sites are marked according to the dose adjustment proposed in Table 3. A complementary dose can be administered 15 days later if residual spasm or asymmetry is present.
CONCLUSION
The technique proposed for the bilateral application of botulinum toxin was effective in controlling HFS and prevented iatrogenic asymmetry of the facial muscles, a complication observed in cases in which unilateral application is used to solely treat dystonia.
There was a statistically significant difference between the doses applied to each side of the face with regard to the zygomaticus, orbicularis oris, and orbicularis oculi muscles. The analysis should be performed on a case-by-case basis, but in general, the toxin should be applied at a 1:1.5 U ratio at the orbicularis oculi (lateral portion) and a 1:2 U ratio at the orbicularis oris. In the remaining muscles (frontal, corrugator, depressor labii inferioris, levator labii superioris, and risorius), the same dose should be applied on both sides. The zygomaticus should receive a similar dose, and a complementary dose can be administered 15 days later if there is some degree of spasm. There is the option of using an asymmetrical dose of 1:1.5 U if the spasm is very intense in this area. The pretarsal region of the orbicularis oculi is the only area for which botulinum toxin application on the healthy side is unnecessary.
There was a low incidence of complications and these were mild and self-limited. The cost of a mean dose of 48.6 U per session is feasible, considering that the mean annual cost of the medication to treat one patient was 804.13 R$.
REFERENCES
1. Wilkins RH. Hemifacial spasm: a review. Surg Neurol. 1991;36(4):251-77. PMID: 1948626 DOI: http://dx.doi.org/10.1016/0090-3019(91)90087-P
2. Tan NC, Chan LL, Tan EK. Hemifacial spasm and involuntary facial movements. QJM. 2002;95(8):493-500. PMID: 12145388 DOI:http://dx.doi.org/10.1093/qjmed/95.8.493
3. Holds JB, Alderson K, Fogg SG, Anderson RL. Motor nerve sprouting in human orbicularis muscle after botulinum A injection. Invest Ophthalmol Vis Sci. 1990;31(5):964-7.
4. Horn AK, Porter JD, Evinger C. Botulinum toxin paralysis of the orbicularis oculi muscle. Types and time course of alterations in muscle structure, physiology and lid kinematics. Exp Brain Res. 1993;96(1):39-53. DOI: http://dx.doi.org/10.1007/BF00230437
5. Elston SJ. Botulinum toxin treatment of hemifacial spasm. J Neurol Neurosurg Psychiatry. 1986;49(7):827-9. DOI:http://dx.doi.org/10.1136/jnnp.49.7.827
6. Flanders M, Chin D, Boghen D. Botulinum toxin: preferred treatment for hemifacial spasm. Eur Neurol. 1993;33(4):316-9. PMID:8348919 DOI: http://dx.doi.org/10.1159/000116961
7. Bentivoglio AR, Fasano A, Ialongo T, Soleti F, Lo Fermo S, Albanese A. Outcome predictors, efficacy and safety of Botox and Dysport in the long-term treatment of hemifacial spasm. Eur J Neurol. 2009;16(3):392-8. DOI: http://dx.doi.org/10.1111/j.1468-1331.2008.02507.x
8. Kollewe K, Mohammadi B, Dengler R, Dressler D. Hemifacial spasm and reinnervation synkinesias: long-term treatment with either Botox or Dysport. J Neural Transm. 2010;117(6):759-63. PMID:20437061 DOI: http://dx.doi.org/10.1007/s00702-010-0409-4
9. Ortisi E, Henderson HW, Bunce C, Xing W, Collin JR. Blepharospasm and hemifacial spasm: a protocol for titration of botulinum toxin dose to the individual patient and for the management of refractory cases. Eye (Lond). 2006;20(8):916-22. DOI:http://dx.doi.org/10.1038/sj.eye.6702054
10. Park YC, Lim JK, Lee DK, Yi SD. Botulinum a toxin treatment of hemifacial spasm and blepharospasm. J Korean Med Sci. 1993;8(5):334-40. DOI: http://dx.doi.org/10.3346/jkms.1993.8.5.334
11. Clark RP, Berris CE. Botulinum toxin: a treatment for facial asymmetry caused by facial nerve paralysis. Plast Reconstr Surg. 1989;84(2):353-5. PMID: 2748749 DOI:http://dx.doi.org/10.1097/00006534-198908000-00027
12. Oyama H, Ikeda A, Inoue S, Nakashima Y, Shibuya M. Local injection of botulinum toxin type A for hemifacial spasm. Neurol Med Chir (Tokyo). 2002;42(6):245-8. DOI:http://dx.doi.org/10.2176/nmc.42.245
13. Borodic GE, Cheney M, McKenna M. Contralateral injections of botulinum A toxin for the treatment of hemifacial spasm to achieve increased facial symmetry. Plast Reconstr Surg. 1992;90(6):972-7. DOI: http://dx.doi.org/10.1097/00006534-199212000-00004
14. Defazio G, Abbruzzese G, Girlanda P, Vacca L, Currà A, De Salvia R, et al. Botulinum toxin A treatment for primary hemifacial spasm: a 10-year multicenter study. Arch Neurol. 2002;59(3):418-20. PMID:11890846 DOI: http://dx.doi.org/10.1001/archneur.59.3.418
15. Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55(1):8-14. PMID: 20418969 DOI: http://dx.doi.org/10.4103/0019-5154.60343
16. Dhaked RK, Singh MK, Singh P, Gupta P. Botulinum toxin: bioweapon & magic drug. Indian J Med Res. 2010;132:489-503. PMID:21149997
17. Kenney C, Jankovic J. Botulinum toxin in the treatment of blepharospasm and hemifacial spasm. J Neural Transm. 2008;115(4):585-91. PMID: 17558461 DOI:http://dx.doi.org/10.1007/s00702-007-0768-7
18. Frei K, Truong DD, Dressler D. Botulinum toxin therapy of hemifacial spasm: comparing different therapeutic preparations. Eur J Neurol. 2006;13 Suppl 1:30-5. DOI: http://dx.doi.org/10.1111/j.1468-1331.2006.01442.x
19. Singer C, Papapetropoulos S, Farronay O. Childhood-onset hemifacial spasm: successful treatment with botulinum toxin. Pediatr Neurol. 2005;33(3):220-2. PMID: 16139741 DOI:http://dx.doi.org/10.1016/j.pediatrneurol.2005.03.008
20. Eleopra R, Tugnoli V, Caniatti L, De Grandis D. Botulinum toxin treatment in the facial muscles of humans: evidence of an action in untreated near muscles by peripheral local diffusion. Neurology. 1996;46(4):1158-60. DOI: http://dx.doi.org/10.1212/WNL.46.4.1158
21. Salles AG. Avaliação do efeito da toxina botulínica no lado são em pacientes com paralisia facial de longa duração. [Tese de doutorado]. São Paulo: Universidade de São Paulo, Faculdade de Medicina, 2006. 94p.
22. Salles AG, Toledo PN, Ferreira MC. Botulinum toxin injection in long-standing facial paralysis patients: improvement of facial symmetry observed up to 6 months. Aesthetic Plast Surg. 2009;33(4):582-90. PMID: 19330369 DOI:http://dx.doi.org/10.1007/s00266-009-9337-9
23. Yang SS, Seet RC, Lim EC. Action-induced hemifacial spasm and its resolution with botulinum toxin. Mov Disord. 2009;24(1):147-8. DOI:http://dx.doi.org/10.1002/mds.22338
24. Barbosa ER, Takada LT, Gonçalves LR, Costa RM, Silveira-Moriyama L, Chien HF. Botulinum toxin type A in the treatment of hemifacial spasm: an 11-year experience. Arq Neuropsiquiatr. 2010;68(4):502-5. PMID: 20730300 DOI:http://dx.doi.org/10.1590/S0004-282X2010000400006
25. Wabbels B, Roggenkämper P. Botulinum toxin in hemifacial spasm: the challenge to assess the effect of treatment. J Neural Transm. 2012;119(8):963-80. PMID: 22231846 DOI:http://dx.doi.org/10.1007/s00702-011-0762-y
26. Rudzińska M, Wójcik M, Szczudlik A. Hemifacial spasm non-motor and motor-related symptoms and their response to botulinum toxin therapy. J Neural Transm. 2010;117(6):765-72. DOI:http://dx.doi.org/10.1007/s00702-010-0416-5
27. Vogt T, Lüssi F, Paul A, Urban P. Long-term therapy of focal dystonia and facial hemispasm with botulinum toxin A. Nervenarzt. 2008;79(8):912-7. PMID: 18551268 DOI:http://dx.doi.org/10.1007/s00115-008-2486-2
28. Meunier FA, Lisk G, Sesardic D, Dolly JO. Dynamics of motor nerve terminal remodeling unveiled using SNARE-cleaving botulinum toxins: the extent and duration are dictated by the sites of SNAP-25 truncation. Mol Cell Neurosci. 2003;22(4):454-66. DOI:http://dx.doi.org/10.1016/S1044-7431(02)00016-7
29. Colakoglu BD, Cakmur R, Uzunel F. Is it always necessary to apply botulinum toxin into the lower facial muscles in hemifacial spasm?: a randomized, single-blind, crossover trial. Eur Neurol. 2011;65(5):286-90. DOI: http://dx.doi.org/10.1159/000327534.
Universidade de São Paulo, São Paulo, SP, Brazil
Institution: Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil.
Corresponding author:
Alessandra Grassi Salles
Rua Joaquim Floriano, 466, cj. 2102
São Paulo, SP, Brazil Zip code 04534-002
E-mail: agsalles@uol.com.br
Article received: July 20, 2014.
Article accepted: April 21, 2015.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter