

Original Article - Year 2015 - Volume 30 -
Use of a transverse submandibular cervical flap in the repair of defects of the middle third of the face
Uso do retalho cérvico-submandibular transverso nas reparações de defeitos do terço médio da face
ABSTRACT
INTRODUCTION: The need for large amounts of skin and subcutaneous tissue makes it complex to repair extensive defects of the middle third of the face. Aiming to reduce morbidity and attain good aesthetic-functional outcomes, Ariyan and McGrath and, subsequently, Behan et al. proposed reconstruction techniques that use transverse and submandibular cervical flap, respectively. Such flaps have fair amounts of tissue and are associated with low morbidity in the donor area. The present authors propose and describe a variant of the Behan flap for use in the reconstruction of large defects of the middle third of the face.
METHODS: We conducted a retrospective study of 8 cases of reconstruction with transverse submandibular cervical flaps for defects of the middle third of the face, conducted between June 2011 and December 2013. The following parameters were analyzed: possible results, and early and late complications.
RESULTS: Eight patients with a mean age of 73.5 years were included. All of the patients presented aesthetic-functional satisfactory results. Among the early complications, vascular congestion occurred in 3 patients in the first week with spontaneous resolution, and salivary fistula occurred after tumor resection in 1 patient. With regard to late complications, scar retraction was observed, manifested by either by ectropion (2 patients) or labial retraction (1 patient).
CONCLUSION: Randomized transverse submandibular cervical flaps with axial pedicles may be considered as another option for reconstruction of defects of the middle third of the face.
Keywords: Surgical flaps; Head and neck cancer; Reconstruction; Plastic surgery; Elderly; Reconstructive surgical procedures.
RESUMO
INTRODUÇÃO: As reparações de defeitos extensos do terço médio da face, ao necessitarem de grandes quantidades de pele e subcutâneo, tornam-se complexas. Buscando reduzir morbidade e associar bom resultado estético-funcional, Ariyan & McGrath e, posteriormente, Behan et al. propuseram reconstruções a partir de retalhos transversos cervicais e cérvico-submandibulares, respectivamente. Tais retalhos dispõem de boas quantidades de tecidos e baixa morbidade da área doadora. Os autores propõem e descrevem uma variante do retalho de Behan para reconstruções de grandes defeitos no terço médio de face.
MÉTODOS: Foi realizado um estudo retrospectivo de 8 casos de reconstrução do terço médio da face pelo retalho cérvico-submandibular transverso, no período de junho de 2011 a dezembro de 2013. Os parâmetros analisados foram: resultados possíveis e complicações precoces e tardias.
RESULTADOS: Foram operados 8 pacientes, com média de idade de 73,5 anos. Todos os pacientes apresentaram resultados estético-funcionais satisfatórios. Dentre as complicações precoces, 3 pacientes apresentaram congestão vascular na primeira semana com resolução espontânea e 1 apresentou fistula salivar consequente à ressecção tumoral. Em relação às complicações tardias, a retração cicatricial foi a complicação observada, manifestada por ectrópio (2 pacientes) e retração labial (1 paciente).
CONCLUSÃO: O retalho cérvico-submandibular transverso randomizado e com pedículos axiais é mais uma opção para reconstruções de defeitos do terço médio da face.
Palavras-chave: Retalhos cirúrgicos; Neoplasias de cabeça e pescoço; Reconstrução; Cirurgia plástica; Idoso; Procedimentos cirúrgicos reconstrutivos.
The repair of extensive defects in the middle third of the face is complex because of the need for large amounts of skin and subcutaneous tissue. It is frequently required in resections of advanced neoplasms, which generally affect elderly individuals, with several comorbidities. Consequently, the lowest possible morbidity, aesthetic-functional outcome, and satisfactory resolution in a single surgery should be aimed for as much as possible in repair of defects.
Based on prior anatomical studies on the cervical region1 and the demonstration of the territory of vascular perforators from the external carotid artery performed by Imanishi et al.,2 Behan et al.3,4 described a flap irrigated from these perforating vessels, arranged in a transverse manner in the submandibular region. The Behan flap is not a true axial flap, and the skeletonization of a specific perforator is not convenient 4. This is one alternative to microsurgical flaps, which is associated with low morbidity in the donor area and good skin resemblance to the middle third of the face.
The planning for the use of this type of flap in the cervical region, however, was initially described by McGrath and Ariyan5 in 1977. It was considered, mistakenly, as a platysma myocutaneous flap. This was prepared transversely in the neck, beginning in the lateral cervical region, ipsilateral to the defect. Other publications have proven the effectiveness of this option to repair defects of the head and neck6-8.
OBJECTIVES
The authors propose the use of a variant of the Behan flap to repair defects in the middle third of the face of elderly patients, often with excess skin and subcutaneous tissue in the submandibular region, that are subjected to large resections due to advanced skin neoplasms.
METHODS
In a retrospective study, 8 cases of reconstruction with a transverse submandibular cervical flap for the middle third of the face, conducted between June 2011 and December 2013, were included. The patients underwent operation by the plastic surgery team of the Faculty of Medicine of Hospital de Base de São José do Rio Preto.
The work was approved by the ethics and research committee of the Faculty of Medicine of São José do Rio Preto, under protocol No. 37084114.2.0000.5415.
The following parameters were analyzed: possible results, and early and late complications.
Surgical Technique
After resection of the tumor with sufficient surgical margins, flap placement with an ipsilateral base to the defect was planned. The base of the flap should be positioned on the posterior edge of the middle third of the sternocleidomastoideus muscle. This is where the perforating arteries emerge, the major components of flap irrigation (Figures 1B and 2B).
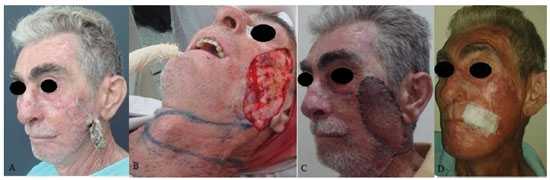
Figure 1. A patient with squamous cell carcinoma in the left middle third of the face. (A) Preoperative aspect. (B) Marking of the predominantly cervical transverse flap. (C) Transposition of the flap with vascular congestion on postoperative day 7. (D) The postoperative aspect at 6 months. Photo sent by the patient with a micropore.
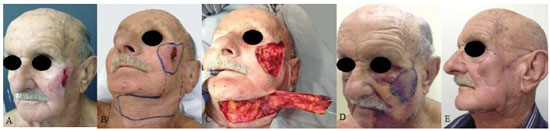
Figure 2. A patient with basal cell carcinoma in the left middle third of the face. (A) Preoperative aspect. (B) Marking of the predominantly submandibular transverse flap. (C) Composition of the flap: skin, subcutaneous tissue, platysma, and deep adipofascial layer. (D) A tunneled flap with vascular congestion on postoperative day 7. (E) Postoperative aspect at 6 months.
The extent of the flap from its base (point of rotation) was then calculated in order to adequately cover the distal portion of the defect. Subsequently, the width of the flap was defined, marking initially the submental crease (anterior margin of the flap). Next, by bidigital clamping, the amount of skin that could be included was verified, and the posterior boundary was defined (Figures 1B and 2B). At this moment, the head should be maintained in neutral craniocaudal position.
The depth of the incision should include the skin, subcutaneous tissue, platysma, and deep adipofascial layer, just below the platysma. All of these structures should be included in the flap. From the contralateral extremity, the flap should be raised to the medial border of the sternocleidomastoid muscle, thereby preserving a sufficient perforator base (Figure 2C). The external jugular vein should be preserved as well. The flap could be transposed (Figures 1, 3, 4 and 7) or tunneled (Figure 2).
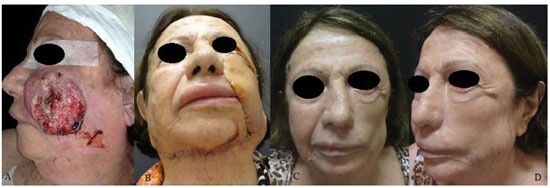
Figure 3. A patient with squamous cell carcinoma in the left middle third of the face. (A) Preoperative aspect. (B) Postoperative aspect on day 7, showing the transposed flap and primary closure of the donor area. (C) and (D) Postoperative aspect at 3 months. Note the ectropion and facial paralysis on the left side.
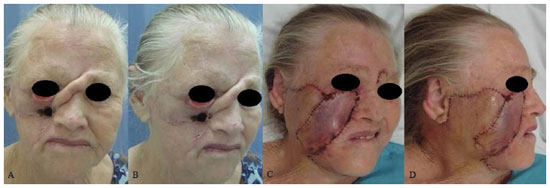
Figure 4. A patient with a paramedian forehead flap and partial necrosis of the Mustardé flap, with exposure of the right maxillary sinus. (A) and B) Preoperative (C) and (D) postoperative aspects of the transposed flap with vascular congestion on day 7.
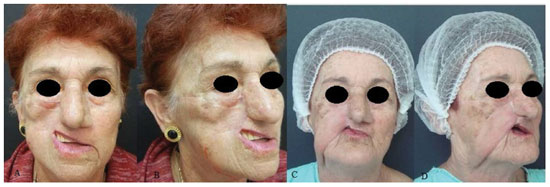
Figure 5. A patient with retraction on the upper right lip after labial reconstruction using a Gillies flap. (A) and (B) Preoperative (C) and (D) postoperative aspects at 6 months.
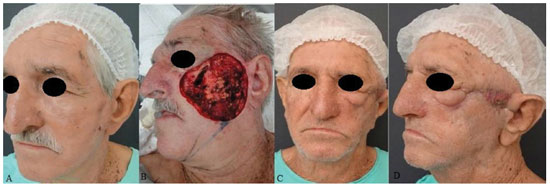
Figure 6. A patient with squamous cell carcinoma in the left middle third of the face. (A) Preoperative aspect. (B) A defect generated after tumor resection. (C) and (D) Postoperative aspects at 12 months of a patient with ectropion and facial paralysis on the left side and recurrent lesion in left temporal region.
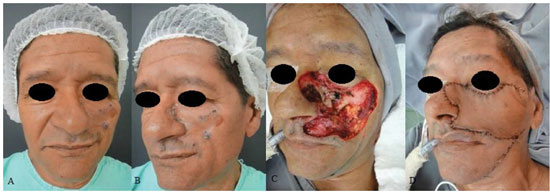
Figure 7. A patient with basal cell carcinoma in the left middle third of the face. (A) and (B) Preoperative aspects. (C) A defect in the middle third of the face resulting from resection of the neoplastic lesion. (D) Immediate postoperative period.
The donor area was primarily closed (Figure 3B), and the flap was positioned at the site to be repaired. Adhesive sutures9 were made between the flap and the receiving area, and a drain in continuous aspiration was left for 24 hours.
RESULTS
Eight patients, 4 men and 4 women, underwent surgery. The age of the patients ranged from 61 to 87 years (mean, 73.5 years). All of the defects corrected were due to neoplasia. Of the patients, 6 underwent immediate reconstruction and 2 presented complications of the first procedure, requiring a new approach.
Among the patients who underwent immediate reconstruction, 4 were histopathologically diagnosed with squamous cell carcinoma and 2 were diagnosed with basal cell carcinoma. Of the patients who underwent a second operation, one presented necrosis of a Mustardé flap after resection of the squamous cell carcinoma, with exposure of the right maxillary sinus. Another presented with scar retraction in the lip, resulting from the implementation of a Gillies flap after resection of a basal cell carcinoma, totaling 8 cases.
The patients were initially followed up according to their needs, which were assessed during the follow-up. After clinical stabilization, follow-up was conducted postoperatively at 1 month, 3 months, and 6 months. The patients were then discharged with referral to the dermatology service for surveillance of new lesions. Late postoperative images were not obtained for 2 cases. One of these patients died, and the other was lost to follow-up.
Among the early complications, congestion occurred in 3 patients, in the first week, with spontaneous resolution (Figures 1C, 2D, 4C, D). One of them had persistent epidermolysis in a small marginal distal segment, without sequelae. One patient had a parotid fistula with spontaneous resolution after 3 weeks. No other early changes were observed.
Three patients presented cicatricial retractions directly related to the procedure, which resulted in a reduction of the lip in one patient and in ectropion in 2 patients (Figures 3C, D and 6C, D). One patient who had had an ectropia related to the first surgery (Figure 4A, B) evolved with recurrence of the neoplastic lesion and infected salivary fistula, with total graft loss at 5 months after the surgery. Loss to follow-up due to death by other causes occurred. Another patient presented labial retraction (Figure 5A, B), also resulting from the first surgery (Table 1).
DISCUSSION
The planning of the use of a transverse flap with a pedicle ipsilateral to the defect to be repaired was first proposed by McGrath and Ariyan in 19785. The flap was composed of skin and cervical subcutaneous tissue, in addition to the platysma muscle. In addition to the positive results presented in a case series, publications explained the anatomy of the cervical region1,10,11, allowing greater safety in the use of flaps to repair defects in the head and neck region.
Anatomical studies have revealed, through cadaver dissections and angiographic examinations, the rich network of blood in the anterolateral region of the neck. The blood supply comes from the submental, facial, upper thyroid, lower thyroid, transverse cervical and occipital arteries, and branches of the external carotid and subclavian arteries. An important detail of this territory is the rich network of perforators of the sternocleidomastoideus4 muscle. Imanishi et al.2 demonstrated that the vascularization of the transverse cervical flap arises from the deep adipofascial layer to the platysma. For this reason, not only the platysma but also this deep structure should be included in the flap. Therefore, the flap has vascular characteristics of a fasciocutaneous, and not a myocutaneous, flap. Behan et al. proposed the implementation of a transverse fasciocutaneous flap, in a similar way to that proposed by McGrath and Ariyan5, except that it is located in the cervical submandibular region. In addition, this flap is an island flap in the form of a keystone12, a curvilinear trapezoidal stone that is fundamental in supporting Roman arches. This flap is supplied by perforators in the middle third of the sternocleidomastoideus muscle. In this article, the flap is described as having the same location as the Behan flap, but not as an island. Thus, irrigation is randomized by the subdermal and axial plexuses based on the perforator veins.
The dual irrigation provides greater safety in preparing flaps with larger dimensions, especially in cases that require overcoming the cervical midline. In general, in case of defects near the central region of the face, this extension becomes necessary, enough to completely cover the defect. In the 3 cases with signs of vascular distress, no correlation was observed with the extension of the flap. Venous congestion was found in the entire flap, with spontaneous resolution after 1 week. No necrosis was observed. Another important aspect is the preservation of the external jugular vein, the main component of venous return13.
The transverse submandibular cervical flap is technically versatile and easy to execute. It presents a short learning curve and discards the need for deep cervical dissection. Its skin color, thickness, and texture are similar to those of the facial skin; thus, the flap shows satisfactory esthetic results. In addition, the adiposity and redundancy of skin in the elderly provides a good amount of available tissue, as well as low morbidity in the donor area. Primary closure is possible, and the scar can be positioned in the submental crease. As it is not an island flap, the flap can be easily transposed and the dog-ear formed at the base of the flap need not be addressed initially. Only one case in our study required posterior correction.
Early complications were self-limited and had good resolution. Besides the patient with transient vascular distress described earlier, another patient had a parotid fistula after surgical excision, which resolved spontaneously in 3 weeks. The case of graft loss occurred after local infection, 5 months after its construction.
Delayed scar retraction directly related to the procedure occurred in 3 patients (Figures 3 and 6). However, in 2 cases, the patients evolved with facial paralysis due to tumor involvement and received postoperative radiotherapy. The direct relationship between intense scar retraction and the proposed flap could not be confirmed, but the authors suggest that the application of the flap should be planned with consideration for a slight safety excess.
CONCLUSION
The transverse submandibular cervical flap, composed of skin, subcutaneous tissue, platysma, and deep adipofascial layer, and vascularized by axial perforating pedicles on the middle third of the sternocleidomastoideus muscle and subdermal plexus, may be considered as an option for reconstruction of defects of the middle third of the face.
REFERENCES
1. Hurwitz DJ, Rabson JA, Futrell JW. The anatomic basis for the platysma skin flap. Plast Reconstr Surg. 1983;72(3):302-14. PMID: 6611752 DOI: http://dx.doi.org/10.1097/00006534-198309000-00005
2. Imanishi N, Nakajima H, Kishi K, Chang H, Aiso S. Is the platysma flap musculocutaneous? Angiographic study of the platysma. Plast Reconstr Surg. 2005;115(4):1018-24. PMID: 15793439 DOI: http://dx.doi.org/10.1097/01.PRS.0000154211.52778.71
3. Behan F, Sizeland A, Gilmour F, Hui A, Seel M, Lo CH. Use of the keystone island flap for advanced head and neck cancer in the elderly--a principle of amelioration. J Plast Reconstr Aesthet Surg. 2010;63(5):739-45. DOI: http://dx.doi.org/10.1016/j.bjps.2009.01.079
4. Behan FC, Rozen WM, Wilson J, Kapila S, Sizeland A, Findlay MW. The cervico-submental keystone island flap for locoregional head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2013;66(1):23-8. DOI: http://dx.doi.org/10.1016/j.bjps.2012.08.027
5. McGrath MH, Ariyan S. Immediate reconstruction of full-thickness burn of an ear with an undelayed myocutaneous flap. Case report. Plast Reconstr Surg. 1978;62(4):618-21. PMID: 358240
6. Ariyan S. The transverse platysma myocutaneous flap for head and neck reconstruction. Plast Reconstr Surg. 1997;99(2):340-7. DOI: http://dx.doi.org/10.1097/00006534-199702000-00006
7. Peng LW, Zhang WF, Zhao JH, He SG, Zhao YF. Two designs of platysma myocutaneous flap for reconstruction of oral and facial defects following cancer surgery. Int J Oral Maxillofac Surg. 2005;34(5):507-13. DOI: http://dx.doi.org/10.1016/j.ijom.2004.10.022
8. Su T, Zhao YF, Liu B, Hu YP, Zhang WF. Clinical review of three types of platysma myocutaneous flap. Int J Oral Maxillofac Surg. 2006;35(11):1011-5. PMID: 17000080 DOI: http://dx.doi.org/10.1016/j.ijom.2006.08.002
9. Baroudi R, Ferreira CA. Seroma: how to avoid it and how to treat it. Aesthet Surg J. 1998;18(6):439-41. PMID: 19328174 DOI: http://dx.doi.org/10.1016/S1090-820X(98)70073-1
10. Uehara M, Helman JI, Lillie JH, Brooks SL. Blood supply to the platysma muscle flap: an anatomic study with clinical correlation. J Oral Maxillofac Surg. 2001;59(6):642-6. PMID: 11381387 DOI: http://dx.doi.org/10.1053/joms.2001.23389
11. Coleman JJ 3rd, Jurkiewicz MJ, Nahai F, Mathes SJ. The platysma musculocutaneous flap: experience with 24 cases. Plast Reconstr Surg. 1983;72(3):315-23. PMID: 6611753 DOI: http://dx.doi.org/10.1097/00006534-198309000-00007
12. Behan FC. The Keystone Design Perforator Island Flap in reconstructive surgery. ANZ J Surg. 2003;73(3):112-20. PMID: 12608972 DOI: http://dx.doi.org/10.1046/j.1445-2197.2003.02638.x
13. Baur DA, Helman JI. The posteriorly based platysma flap in oral and facial reconstruction: A case series. J Oral Maxillofac Surg. 2002;60(10):1147-50. DOI: http://dx.doi.org/10.1053/joms.2002.34989
1. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
2. Faculdade de Medicina de São José do Rio Preto, São José do Rio Preto, SP, Brazil
Institution: Hospital de Base da Faculdade de Medicina de São José do Rio Preto - FAMERP, São José do Rio Preto, SP, Brazil.
Corresponding author:
Antônio Roberto Bozola
Av. Brigadeiro Faria Lima, 5.416, Vila São Pedro
São José do Rio Preto, SP, Brazil Zip Code 15090-000
E-mail: ceplastica@hotmail.com
Article received November 24, 2014.
Article accepted April 21, 2015.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter