

Reviw Article - Year 2015 - Volume 30 -
Late seroma in breast reconstructions and mammoplasty with silicone implants: a case report and literature review
Seroma tardio em reconstruções mamárias e mamoplastias com implante de silicone: relato de caso e revisão da literatura
ABSTRACT
The occurrence of seroma as a late complication of silicone breast implant is of great interest, given the aesthetic implications such as asymmetry and the possible association with infections or even malignancies. This complication is believed to be exclusive of textured prostheses. The present authors reviewed the literature by conducting a search of data in publications available in Medline by using the search term "late breast seroma" in order to clarify the pathological features of seroma. The etiology is unknown in most cases. The definitive treatment of choice is surgery, and most authors recommend bacteriological and cytological evaluations for seroma, preferably guided by ultrasonography. To provide patients with the best treatment, the treatment should be individualized according to clinical presentation, anticipating the possibility of recurrence and final sequelae.
Keywords: Prostheses and implants; Postoperative complications; Breast Augmentation; Breast reconstruction; Seroma; Recurrence.
RESUMO
A ocorrência de seroma como complicação tardia por implante de silicone mamário é de grande interesse, dadas as implicações estéticas, como assimetria, e a possível associação com infecções ou até mesmo malignidades. Acredita-se que esta complicação seja exclusiva de próteses texturizadas. Os autores fazem síntese da literatura a partir de pesquisa de dados em publicações disponíveis em MEDLINE com o termo "late breast seroma" em busca de maior esclarecimento da patologia. A etiologia é desconhecida na maioria dos casos. Nota-se que o tratamento definitivo de escolha é cirúrgico, sendo que grande parte dos autores recomenda a avaliação bacteriológica e citológica do seroma, preferencialmente guiado por ultrassonografia. O tratamento deve ser individualizado, de acordo com a clínica apresentada, antecipando a possibilidade de recorrência do evento e a sequela final, oferecendo, assim, o melhor tratamento à paciente.
Palavras-chave: Próteses e implantes; Complicações pós-operatórias; Mamoplastia; Reconstrução da mama; Seroma; Recidiva.
Late seroma is classified as clinical evidence of seroma (mammary swelling), without the observation of documented infection, more than a year after breast implantation1,2. It can also be defined from 3 months after breast augmentation, according to the International Society of Cosmetic Surgery3. The issue is seldom discussed in the literature, does not have a defined etiology, and surrounded by large speculations1. Although the occurrence of late seroma is usually related to micro traumas and subclinical infections, a rare association with large-cell anaplastic lymphoma, a type of non-Hodgkin lymphoma of the breast, has been discussed1,2.
The incidence of late seroma ranges from 1% to 2%4 in most studies. In the event of fluid collections with an increase in breast volume, the presence of infection must first be ruled out2. Then, folds in the prosthesis, irritation by friction, or allergic phenomena should be considered4. In the occurrence of early seroma beginning at 6 months after surgery, experts now recommend diagnostic assessment, especially if the seroma is recurrent5.
The numbers of reports on late seroma and hematomas have increased with the increased use of textured implants, which are used to reduce the incidence of capsular contracture. The diversity of variables such as the texture and positioning of implants hinders the analysis of data and the development of an algorithm for its analysis. Given the small number of studies with large samples, this article proposes to review the already established knowledge on this complication to better guide plastic surgeons.
METHODS
This is a review article on late seroma related to the use of silicone breast implants, with a search of data in publications from the last 5 years, which are available in the databases of Medline, by using the search term "late breast seroma". Eighteen publications were selected, in which 76 cases were analyzed, encompassing the clinical-surgical approach to the disorder, in addition to the theories proposed for its etiology, which is still unknown. An article published 10 years ago was included because of its relevance to the issue.
RESULTS
In the literature review, 76 reports on late seroma that presented between 16 months and 10 years after breast implant surgery, either for reconstruction after mastectomy or for aesthetic purposes, were assessed. The latter purpose predominated in this study, corresponding to 81.57% of all surgeries performed, encompassing both augmentation mammoplasties and mastopexy with prosthesis. The implants related to late seroma were textured. Only one event was related to the use of smooth prosthesis (1.3%), without associated triggering factors.
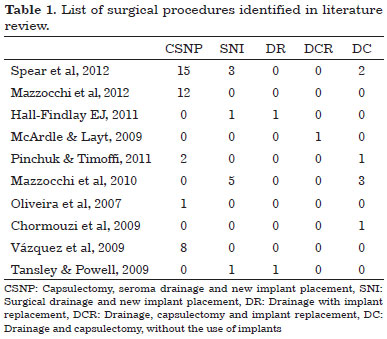
Of the 76 patients described, 35 underwent initial drainage of serous fluid, preferably guided by ultrasonography, but relapsed, requiring new drainage or surgical procedures1,4,6-8. After recurrence, surgical procedures were adopted by most of the authors. Definitive conservative treatment, including evacuation of liquid via capsular centesis and clinical treatment only, with antibiotics, anti-inflammatory agents, and even antiviral agents, was performed successfully in 23.68%. Various surgical procedures were applied definitely in 76.32% of the patients and are described in Table 1.
DISCUSSION
Etiopathogenesis
Periprosthetic fluid collection is idiopathic in most cases2. Several propositions to explain the pathophysiological mechanism of late seroma were formulated, none of which provided a satisfactory explanation. In suspected cases of non-Hodgkin lymphoma, B symptoms such as fever, night sweats, and weight loss, in addition to cloudy periprosthetic seroma, are observed. It is believed that this type of lymphoma has a more benign course and can be treated effectively by the drainage of the seroma and removal of the implants, without needing chemotherapy or radiotherapy. These recommendations are based on empirical observations and still lack more robust studies9.
Late seroma is believed to be a unique complication of textured implants, due to tissue irritation caused by the greater contact area of the textured surface7,10. In some patients, upon surgical exploration, two fibrous capsules are observed, with the inner layer adhering firmly to the prosthesis and the outer layer adhering to the breast tissue. The potential space between the two layers2 and the shear forces between them would trigger seroma formation11. In cases related to smooth-surface implants, other risk factors were present, such as the use of corticosteroids, traumas, or micro ruptures in the implant4.
The shear forces acting between the surfaces of the implant and the adjacent tissues induce the formation of synovial metaplasia in the capsule2. The liquid formed to slide between the surfaces is considered normal and does not justify the clinical unilateral and late breast augmentation in patients with implants12. Vazquez et al.8, in 2011, suggested that the occurrence of periprosthetic micro ruptures in the capsule would be necessary for the formation of inflammatory exudate. This was concluded according to the higher incidence of late seroma in the right breasts in the authors' study, the dominant limb, and greater movement in most of the patients.
Farina Jr et al.13 related the occurrence of unilateral seroma with micro trauma caused by sports. In this case, conservative treatment with rest and administration of anti-inflammatory and antibiotic therapy was effective in two occasions in the same patient. The patient was instructed to use firmer clothing during sports as prophylaxis, in order to avoid the shearing forces supposedly involved in the pathophysiological mechanism of late seroma. No recurrence was observed after 4 years of follow-up13. Subclinical infections in the form of biofilms were also suggested as possible causes of seroma. Pajkos et al.14 showed that Staphylococcus epidermidis could remain for years after the inclusion of the implant, without clinical signs of infection. The extracellular matrix of this biofilm would be responsible for the increase in bacterial resistance to antibiotics, and their activation could be precipitated by a decline in the immune status of the patient, thus justifying the prodrome with viral or bacterial infections noted in their study.
This hypothesis can be accepted for cases treated only conservatively, as the immune system would be able to recover and inhibit such bacterial activation. Pinchuk and Tymofii12 evaluated a patient who presented with late seroma bilaterally, with prodromal flu. As the patient had no pain or breast deformities, treatment with acyclovir was administered and was successful. In this study, patients with capsular contracture or folds in the associated implants were treated with capsulectomy, drainage of the seroma, and implant replacement, all in the same former pocket, with the exception of one patient who had only mastopexy without the positioning of new prosthesis, according to the patient's preference. No complications were observed during the follow-up period, which ranged from 3 months to 1 year.
Propaedeutics
Magnetic resonance imaging is the most accurate method to study periprosthetic fluid collections and their characteristics, and to detect implant ruptures, the presence of silicone gel outside the capsule, and deformities in its contour2,10. With respect to the diagnosis of lymphoproliferative disorders, the role of imaging examinations has not been defined yet, and cytological puncture is necessary for its diagnostic examination15. The use of computed tomography has also been suggested as an alternative method. However, breast ultrasonography still has advantages such as its low cost and capability of guiding the liquid through the puncture for sampling, in addition to differentiating generalized breast edema from fluid collections2. Evaluation of acute mammary asymmetry through mammography is believed to present a greater potential for damage than for benefits, as it can be painful and create a surgical emergency in the case of late hematoma2.
Treatment
Maintaining a good physician-patient relationship is important when facing such a complication. Spear et al.1 reported that among 28 patients who presented late seroma, one refused to undergo the indicated surgical intervention and was subjected to antibiotic therapy instead in three regimens, with good resolution of symptoms each time. Her last visit was 4 months after the last course of the antibiotic therapy, with complete remission until then.
Although strict clinical treatment was administered in 6 of the 76 cases in this study (Figure 1), infectious or malignant processes should be excluded prior to the commencement of the treatment, through a guided puncture for sample collection for culture and cytological examination.
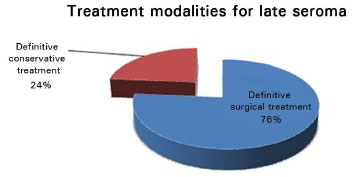
Figure 1. Definitive treatment modalities for late seroma.
The priority of most of the authors was to reduce the risk of new interventions and possible aesthetic deformities. Evidence of infection or masses suggestive of neoplastic involvement is indication for a direct open surgical approach. In the absence of this evidence, ultrasonography-guided puncture should be performed, with culture and cytological examination of the collected sample15. Mazzochi et al.4 observed recurrence of seroma in all patients evaluated by puncture, which led them to establish the removal of the affected prosthesis and perform total capsulectomy and laboratory assessment of the capsule and the serous fluid as the definitive treatment. Whenever possible, the implant pocket was changed, from retroglandular to retromuscular. When no such option is available, a new prosthesis was positioned after a 6-month interval. The replacement of a textured prosthesis with a smooth-surface or polyurethane prosthesis is also one proposal4 but is not implemented in practice by most authors in order to maintain contralateral breast symmetry4,12. In the follow-up period of 1 year, the surgical results were unchanged in the patients who underwent implant replacement4.
The completion of capsulectomy may be important for the reduction of dead space after prosthesis withdrawal. In the case of thin capsules, collapse is possible, which can reduce the dead space. However, thick-walled capsules may remain fixed in their position after the implant replacement, leading to a potential space for seroma formation.
Bengtson et al.2, in their algorithm, guide the inspection of the capsule in the perioperative period and biopsy in areas of abnormal aspects such as thickening and nodularity. The following recommendations were derived from their study: removal of the affected implants and capsules, irrigation of the pocket with antibiotics before the placement of a new implant (this is optional), and empirical antibiotic therapy for subclinical infections with negative culture results. Complete capsulectomy may be reserved for cases refractory to treatment only if a risk of damage to adjacent structures during withdrawal is present. Complications such as capsular contracture are known to be more frequent in patients undergoing partial capsulectomy, and sterile collections are rarely resolved without total capsulectomy. The change in the position of the prosthesis or the type of prosthesis to be positioned was not mentioned in this study2.
The importance of different implant pockets and the types of implants used remains controversial1. Hall-Findlay16 reported having observed late seroma only after using textured prostheses (Biocell® textured implants). For its treatment, the author only released the internal capsule formed adherently to the prosthesis, positioning the same implant in the pocket created in the primary surgery, as no signs of local infection were observed. However, electron microscopy of the implant revealed the presence of a biofilm that resembled Staphylococcus epidermidis. Its presence alone did not justify seroma formation, as the biofilm can be formed around all types of prostheses, but the seroma was only observed in Biocell® implants. Thus, this author relates the probable occurrence of late seroma to a mechanical factor16.
Spear et al.1 also reported the occurrence of late seroma only in textured Biocell® prostheses. However, this was the only type of textured prosthesis used by surgeons involved in the study; thus, the conclusions on the prostheses' role in the pathophysiological mechanism of late seroma cannot be generalized. No recurrence was observed in the patients who underwent replacement of prostheses with either a smooth or textured Biocell®1 prosthesis This study also draws attention to the types of recommended cytopathological examinations, as the routine cultures usually implemented do not seem to be sufficiently sensitive or specific to detect chronic infections by biofilms1. It is important to stress that cultures rarely yielded positive results in the studies analyzed in this review, when conventional examinations were performed12,17. Djedovic et al.18 highlighted the so-called seroma with low-grade or subclinical infection. The sensitivity to detect this would be greater by centrifuged lavage culture, which leads to detachment of bacteria adhered to the breast implant, a technique already presented by Pajkos et al.14 for use in breasts affected by capsular contracture. As such, it would be essential to remove the prosthesis from contact with the late seroma18, not repositioning it after simple drainage of liquid and washing7,16. Bengtson et al.2 also suggested a cytological evaluation with immunohistochemical analysis of CD30 and cytokeratin expressions for cases of seroma with cytological examination results positive for malignancy.
CONCLUSION
The occurrence of late seroma in patients who received silicone implants, whether for reconstructive or aesthetic motivations, is increasingly being reported in the literature. Clinical seromas have not been observed when processes of exudation and reabsorption of periprosthetic fluid are balanced; this process being considered normal. The triggering factors of imbalance of the shear forces in the late postoperative period remain unclear. Studies with a large number of patients are still limited for defining protocols regarding this affliction. The forms of treatment should be individualized according to clinical presentation, including the risk of infection or neoplastic processes, the recurrence of the event, and the final sequela.
REFERENCES
1. Spear SL, Rottman SJ, Glicksman C, Brown M, Al-Attar A. Late seromas after breast implants: theory and practice. Plast Reconstr Surg. 2012;130(2):423-35. PMID: 22495216 DOI: http://dx.doi.org/10.1097/PRS.0b013e3182589ea9
2. Bengtson B, Brody GS, Brown MH, Glicksman C, Hammond D, Kaplan H, et al.; Late Periprosthetic Fluid Collection after Breast Implant Working Group. Managing late periprosthetic fluid collections (seroma) in patients with breast implants: a consensus panel recommendation and review of the literature. Plast Reconstr Surg. 2011;128(1):1-7. PMID: 21441845 DOI: http://dx.doi.org/10.1097/PRS.0b013e318217fdb0
3. Gulyás G. Commentary on "Seroma as a late complication after breast augmentation" by V.D. Pinchuk, O.V. Tymofii. Aesthetic Plast Surg. 2011;35(3):315-8. DOI: http://dx.doi.org/10.1007/s00266010-9607-6
4. Mazzocchi M, Dessy LA, Corrias F, Scuderi N. A clinical study of late seroma in breast implantation surgery. Aesthetic Plast Surg. 2012;36(1):97-104. DOI: http://dx.doi.org/10.1007/s00266-011-9755-3
5. Murphy S, Carroll S. Importance of histological analysis of seroma fluid. Aesthetic Plast Surg. 2013;37(1):187-8. DOI: http://dx.doi.org/10.1007/s00266-012-0007-y
6. Mazzocchi M, Dessy LA, Carlesimo B, Marchetti F, Scuderi N. Late seroma formation after breast surgery with textured silicone implants: a problem worth bearing in mind. Plast Reconstr Surg. 2010;125(4):176e-177e. DOI: http://dx.doi.org/10.1097/PRS.0b013e3181cb664d
7. McArdle B, Layt C. A case of late unilateral hematoma and subsequent late seroma of the breast after bilateral breast augmentation. Aesthetic Plast Surg. 2009;33(4):669-70. DOI: http://dx.doi.org/10.1007/s00266-009-9325-0
8. Vázquez G, Audoin F, Pellón A. Los microtraumatismos como etiologia del seroma tardio en la mamoplastia de aumento. Cir Plást Iberolatinoam. 2011;37(3):215-22. DOI: http://dx.doi.org/10.4321/S0376-78922011000300002
9. Chung KC. Discussion: Managing late periprosthetic fluid collections (seroma) in patients with breast implants: a consensus panel recommendation and review of the literature. Plast Reconstr Surg. 2011;128(1):13-6. PMID: 21701294 DOI: http://dx.doi.org/10.1097/PRS.0b013e31821cf88f
10. Chourmouzi D, Vryzas T, Drevelegas A. New spontaneous breast seroma 5 years after augmentation: a case report. Cases J. 2009;2:7126. DOI: http://dx.doi.org/10.4076/1757-1626-2-7126
11. Robinson HN. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011;128(3):818. PMID: 21866021 DOI: http://dx.doi.org/10.1097/PRS.0b013e3182221513
12. Pinchuk V, Tymofii O. Seroma as a late complication after breast augmentation. Aesthetic Plast Surg. 2011;35(3):303-14. DOI: http://dx.doi.org/10.1007/s00266-010-9607-6
13. Farina JA Jr, Ramalli EL, da Silva MF, Silva R. Jogging as a possible cause of late seroma after aesthetic breast augmentation with textured silicone prosthesis: a conservative approach. J Plast Reconstr Aesthet Surg. 2011;64(8):e216-7. PMID: 21478064 DOI: http://dx.doi.org/10.1016/j.bjps.2011.03.006
14. Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111(5):1605-11. PMID: 12655204 DOI: http://dx.doi.org/10.1097/01.PRS.0000054768.14922.44
15. Tebbetts JB. Diagnosis and management of seroma following breast augmentation: an update. Plast Reconstr Surg. 2011;128(1):17-25. PMID: 21289545 DOI: http://dx.doi.org/10.1097/PRS.0b013e3182134aa3
16. Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconst Surg. 2011;127(1):56-66. PMID: 21200201 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181fad34d
17. Tansley PD, Powell BW. Late swealling after bilateral breast augmentation. J Plast Reconstr Aesthet Surg. 2011;64(2):261-3. PMID: 20434972 DOI: http://dx.doi.org/10.1016/j.bjps.2010.03.037
18. Djedovic G, Pierer G, Rieger UM. Re: Late swelling after bilateral breast augmentation-sonication for detection of subclinical infection. J Plast Reconstr Aesthet Surg. 2011;64(8):1113-4. PMID: 21382758 DOI: http://dx.doi.org/10.1016/j.bjps.2011.02.020
1. Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil
2. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
Institution: Hospital das Clínicas da Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil.
Corresponding author:
Fernanda Dinelli Scala
Rua Rio de Janeiro, 2779, Bairro Lourdes
Belo Horizonte, MG, Brazil Zip Code 30160-042
E-mail: fedinelli@hotmail.com
Article received February 1, 2014.
Article accepted August 3, 2014.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter