

Original Article - Year 2016 - Volume 31 -
Cranioplasties: surgical reconstruction strategies
Cranioplastias: estratégias cirúrgicas de reconstrução
ABSTRACT
INTRODUCTION: Reconstruction of the skull bones can prove challenging. There are three indications for carrying out cranioplasty: (1) recovery of protection against trauma, (2) recovery of the cranial contour, and (3) treatment of the syndrome of the trephined. The objective of this report is to present the experience of the author with cranioplasties, and discuss surgical reconstruction strategies.
METHODS: This report presents a retrospective analysis of 16 consecutive cases of cranial reconstruction, which were operated in 2013 and 2014 in the public health service (INTO - RJ) and in the private practice.
RESULTS: From January 2013 to January 2014, 16 patients underwent surgery. Thirteen were men. Their age ranged from 10 to 72 years. Twelve patients underwent reconstruction with a parietal graft, two with custom prosthesis, one with fracture reduction and fixation, and one with reconstruction of soft parts . Most of the defects were located in the fronto-orbital region. The customized prostheses were used in defects of 192 cm2 and 22.5 cm2. Complications occurred in five patients: lesion of the sagittal sinus, irregularity in the contour, seroma, failure in graft integration, leakage of cerebrospinal fluid, and extrusion of the prosthesis. All the complications were resolved without impairment to the reconstruction. The follow-up time ranged from 10 to 22 months. All the patients were pleased with the reconstructions, and improvement of the neurological functions was reported.
CONCLUSION: Cranioplasty is a primarily restorative surgical procedure that is used to restore the protective function of the skull and to treat the syndrome of the trephined. The autologous parietal graft is the surgeons' first choice. The prosthesis is indicated when there is a major defect or when the harvesting of a parietal graft is not possible.
Keywords: Autografts; Prostheses and implants; Reconstructive surgical procedures; Skull/surgery; Craniotomy.
RESUMO
INTRODUÇÃO: As reconstruções dos ossos do crânio podem ser especialmente desafiadoras. Existem três indicações para se realizar uma cranioplastia: readquirir proteção contra traumas, recuperação do contorno craniano e tratamento da síndrome de trefinado. Este trabalho tem como objetivo mostrar a experiência do autor com cranioplastias e discutir as estratégias cirúrgicas de reconstrução.
MÉTODOS: Foi feita uma análise retrospectiva de 16 casos consecutivos de reconstrução craniana operados na saúde pública (INTO - RJ) e na prática privada em 2013 e 2014.
RESULTADOS: De janeiro de 2013 a janeiro de 2014, 16 pacientes foram operados. Treze eram homens. A idade foi de 10 a 72 anos. Doze pacientes tiveram sua reconstrução feita com enxerto de parietal, 2 com prótese customizada, 1 com redução e fixação da fratura, e 1 com reconstrução de partes moles. A maioria dos defeitos estavam localizados na região fronto-orbital. As próteses customizadas foram usadas em defeitos de 192 e 22,5 cm2. Tivemos complicações em 5 pacientes: lesão de seio sagital, irregularidade no contorno, seroma, não integração do enxerto, vazamento de líquor e extrusão da prótese. Todas as complicações foram resolvidas sem prejuízo à reconstrução. O seguimento variou de 10 a 22 meses. Todos mostraram-se satisfeitos com as reconstruções e houve melhora de funções neurológicas.
CONCLUSÃO: A cranioplastia é uma cirurgia primariamente reparadora para recuperar a função protetora do crânio e tratar a síndrome do trefinado. O enxerto autólogo de parietal segue sendo a primeira escolha. A prótese está indicada quando há um grande defeito ou quando a captação do enxerto parietal não é possível.
Palavras-chave: Autoenxertos; Próteses e implantes; Procedimentos cirúrgicos reconstrutivos; Crânio/cirurgia; Craniotomia.
The reconstructions of the skull bones can prove challenging for a craniofacial plastic surgeon. The defects are often complex, involving the use of other tissues in addition to bone. Furthermore, the proximity to the central nervous system requires technical and anatomic knowledge, and a qualitative surgical material1.
Skull defects might be caused by trauma, a sequela from the neurosurgical intervention, congenital malformation, neoplasia, radiotherapy, and infections2-5. There are basically three indications for carrying out cranioplasty: (1) recovery of the protection against trauma, (2) recovery of the cranial contour, and (3) treatment of the syndrome of the trephined (or sinking skin flap syndrome)6. Cranioplasties can be performed by using autologous bone, allogenic bone (bone bank), or alloplastic materials (hydroxyapatite (HA), titanium, and polymethylmethacrylate). The autologous graft of the parietal external plate is the surgeons' first choice whenever possible. This technique has gained popularity in the 1970s and 1980s with the work of Psillakis & Cardim, in Brazil7, and Tessier, in France8.
The appropriate assessment of the patient is crucial when choosing the surgical reconstruction strategy. The size, nature, and location of the defect; the time interval from the creation of the defect to its correction; the quality of the coverage of the soft parts; the preference of the surgeon; and the history and clinical characteristics of the patient are all aspects that must be taken into account when making the decision to operate9.
OBJECTIVE
The objective of this report is to present the author's personal experience with cranioplasties, and to discuss the surgical reconstruction strategies in accordance with the variables that each case could present.
METHODS
This study is a retrospective analysis of 16 consecutive cases of cranial reconstructions operated in 2013 and 2014 in the public health service (National Institute of Traumatology and Orthopedics - RJ) and in the private practice. The male sex was the most common (n = 13); the patients' age ranged from 10 to 72 years; and the most frequent etiologies were decompressive craniotomy (n = 5) and sequelae from trauma (n = 5).
All these cases were treated by teams in which the author was the first surgeon. The team was comprised of at least one more craniomaxillofacial surgeon and a neurosurgeon.
All the relevant data regarding the patient and the characteristics of the defect, the surgical technique that was used, and the complications that occurred were analyzed and compared with data from published reports. The conduct taken in this work reflects the conduct for cranioplasties of the Center of Specialized Attention of the Craniomaxillofacial Surgery of the National Institute of Traumatology and Orthopedics - RJ, the author's place of training in craniomaxillofacial surgery.
The majority of the cranioplasties were performed by using an autologous graft of the external parietal plate, following the same standard technique whenever possible. Two patients were treated with custom cranial prostheses made with porous ceramic (HA and beta tricalcium phosphate) by Eincobio®, one of them being acquired through donation from the company.
The surgical technique of the parietal graft
All the patients were investigated by using preoperative computerized tomography (CT); they were administered general anesthesia during surgery; they received antibiotic prophylaxis in the first 24 hours postoperatively; and vacuum drainage was used at all the patients' surgical sites. The access of choice was bicoronal, thus allowing for a wide exposure of the defect and of the donor area. The preferred donor area was the posterior parietal bone. The incision was not very anterior. Sometimes we had to ignore the prior incision that was made by the neurosurgeon, and perform another more posterior incision (in such cases one should evaluate the necessity of exposure vs. the risk of necrosis) (Figure 1). For aesthetic reasons, the preference was a Z-shaped incision.
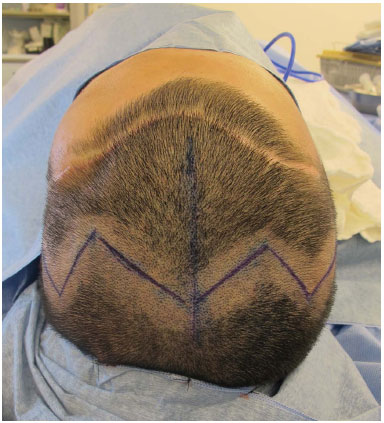
Figure 1. Posterior bicoronal access in the Z-shape ignoring a prior anterior access.
In the loose areolar tissue situated between the galea aponeurotica and the pericranium, the dissection plane was subgaleal. The dissection was performed by using a cold scalpel, which was associated with traction of the flap by the assistant surgeon. This technique allowed for an intact pericranium to be used later as a flap. Often, this is not possible because of previous surgeries. Around the defect and in the donor area, the dissection plan was subperiosteal, thus leaving the bone exposed. In this case, the dissection was performed by using periosteal elevators.
Once the cranial bone defect was delimited, all the tissue immediately above the dura-mater was elevated by using a monopolar electric scalpel at low power (< 30 W). Great care was exercised to ensure the thickest possible coverage of soft parts over the reconstruction. Intimate contact between the dura-mater and the graft was ensured when considering bone integration and revival of the edges of the defect (undertaken by using a drill in order to find the bleeding bone). To minimize bone necrosis, continuous irrigation with saline solution was employed whenever drills and saws were used.
A template of the defect was constructed on the bone fault and transferred to the donor area, usually posterior parietal, thus defining the size and quantity of the grafts. The osteotomies should be performed at a distance of 1.5 cm from the sagittal suture and 1 cm from the coronal suture10 (Figure 2). A drill delimited the grafts until reaching the diploe, when bone bleeding was observed. From there, a sagittal saw was used to cut tangent to the inner plate of the parietal bone in the plane of the diploe, thus releasing the external plate. This was done by peripherally going around the graft, and the central detachment was finalized with a Gigli saw or osteotomes (Figure 3).
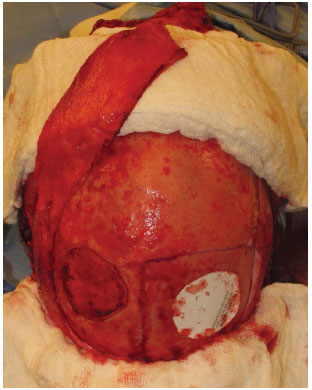
Figure 2. Exposure of the cranial defect, template on the donor area mimicking the defect, sagittal and coronal sutures demarcated for anatomical reference, and prepared pericranium graft.
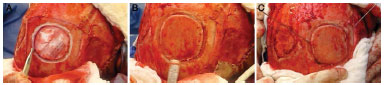
Figure 3. Harvest of the graft of the external parietal plate by using drill, sagittal saw, and a Gigli saw.
With the graft in hand, one had to verify the integrity of the inner plate and whether there was any laceration of the dura-mater. If any leakage of cerebrospinal fluid (CSF) or bleeding from the laceration of the dura-mater was present, we immediately repaired it by using a simple suture or a pericranium patch; the patch could be strengthened with local hemostatic agents, biological glue, or biosynthetic dura-mater, if available.
The graft was then fit onto the defect and fixed. A good fixation and a proper edge-to-edge fit with the revived bone would maximize the connection of the graft. The fixing step was performed by using plates and low profile screws (system < 2.0) or, alternatively, with steel wire no. 0 or 1. A plate with a hole and a bolt on each side was sufficient. Finally, if possible, pericranium grafts were used to cover the rebuilt area, and hemostatic agents were used to cover the donor area (Figure 4). The pericranium grafts, besides providing a structure to nourish the vascularized bone graft, could fill dead spaces and mask any irregularities in the cranial contour. Covering the reconstruction side should be performed without tension and by using viable soft tissues.
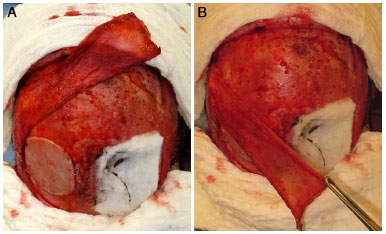
Figure 4. External parietal plate fixed in the cranial defect, donor area covered with hemostatic and pericranium graft covering the rebuilt area and the donor area.
The surgical technique for custom prosthesis
In the event of a custom prosthesis, the procedure was simpler, because there was no donor area. The incision was reduced, aiming only to expose the defect. Sometimes a prior incision could be used (Figure 5). Around the defect, the dissection plane was subperiosteal and the procedure was performed by using periosteum elevators. In the area of the defect itself, the dissection plane was performed by using a monopolar scalpel at low power (< 30 W) just above the dura-mater. Depending on the prosthesis type (porous or not), the revival of the edges of the defect must be done by using a drill to find the bleeding bone to provide bone integration with the material (Figure 6A).
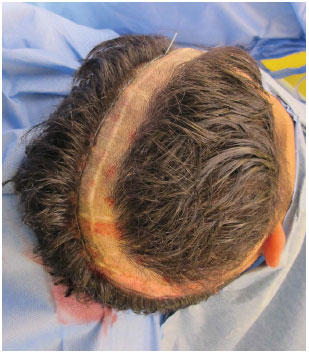
Figure 5. Reduced access by using the previous scar only to expose the defect.
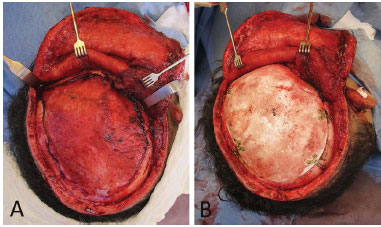
Figure 6. A: Defect exposed with its revived edges; B: Custom prosthesis fixed in the defect. Originally published in: Prototyping: applications in craniomaxillofacial surgery National Institute of Traumatology and Orthopaedics (INTO)- RJ; Maricevich P, Pantoja E, Mansur A, Peixoto A, Amando J, Borges PYV, André Braune A, Nasser JA, Cruz RL. Revista Brasileira de Cirurgia Plástica 30(4)2015.
The prosthesis was fixed by using plates and low profile screws (system < 2.0). Furthermore, the coverage of soft parts must be made by using thick viable tissue and without causing tension. A plate with a hole and a bolt on each side was found adequate. As in most of the cases, we used prostheses on large defects. Further, a point of repair in the dura-mater would prove necessary when attempting to decrease the prospective dead space between the prosthesis and the dura-mater (Figure 6B).
RESULTS
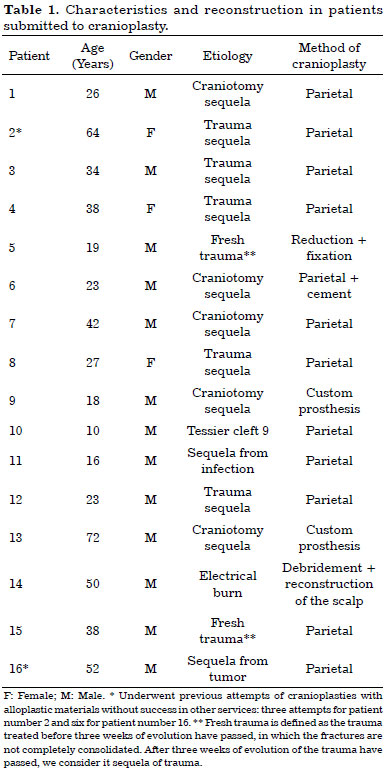
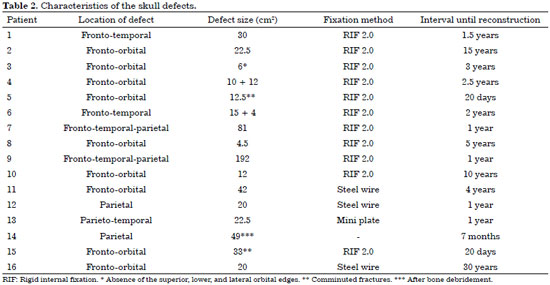
Of the 16 patients who underwent cranioplasty, 12 had undergone reconstructions with autologous parietal grafts, two with custom prosthesis, one with fracture reduction and fixation, and another with reconstruction of the soft parts. In one of the patients who underwent reconstruction with a parietal graft, HA cement was used concomitantly. In two cases, there were previous attempts of cranioplasties with alloplastic materials in other services: three attempts for patient number 2, and six attempts for patient number 16 (Table 1).
All the patients with sequelae showed total thickness defects, except for patient number 14 with sequela from an electrical shock. In this patient, the bone was present, but the tissue was devitalized and presented radiological signs of osteomyelitis. Additionally, patient number 16 had a sequela from a tumor, which was reconstructed once by using a titanium mesh that was found on the verge of extruding. In patient number 14, only debridement of devitalized tissue and reconstruction of the scalp were performed (Figure 7); in patient number 16, the mesh was withdrawn and the parietal bone was used for the reconstruction (Figure 8).
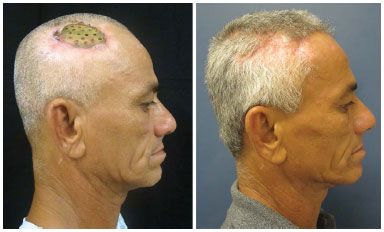
Figure 7. Patient number 14 with exposed parietal bone and radiological signs of osteomyelitis. The bone debridement resulted in a cranial defect of 7 cm x 7 cm. Bone reconstruction was postponed for a second stage, and the reconstruction of the soft parts was carried out using two large rotation flaps of the scalp.
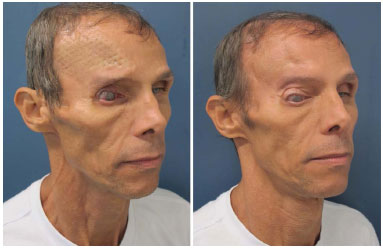
Figure 8. Patient number 16 with a history of six previous attempts of cranioplasties with alloplastic materials that were not successful, presenting a titanium mesh in the imminence of the extrusion. The mesh was removed, and he underwent parietal bone reconstruction.
Seven patients underwent procedures associated with cranioplasties: orbitary reconstruction (sequelae from trauma), resection of the eyelid xanthelasma, treatment of the panfacial fracture (fresh traumas), reconstruction of the upper eyelid (coloboma in cleft 9, Figure 9), and correction of lagophthalmos (arising from an incorrectly positioned titanium mesh).
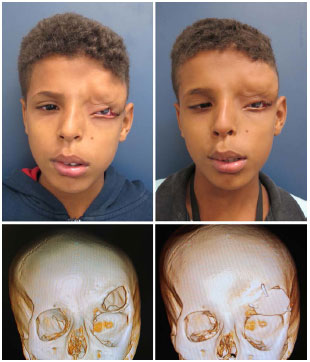
Figure 9. Patient number 10, bearer of Tessier cleft 9, submitted to parietal cranioplasty and reconstruction of the eyelid.
The majority of the defects were located in the fronto-orbital region (n = 9). In patients with fresh trauma of the frontal region, the patency of the nasofrontal duct was attested by CT images (patient number 5), and guaranteed in the transoperative moment through probes (patient number 15). The size of the defect ranged from 4.5 cm2 to 192 cm2, with an average of 39 cm2. The customized prostheses were used in defects of 192 cm2 (Figure 10) and 22.5 cm2. The two custom prostheses were fixed by using the technique of rigid internal fixation (RIF): one by using a 2.0 system and another, a mini plate system. In patient number 5 ( fresh trauma), whose defect was only reduced and fixed, we used RIF 2.0 for fixation.
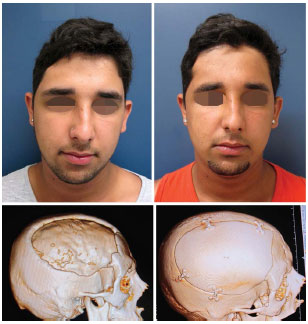
Figure 10. Patient number 9, bearer of cranial defect of 192 cm2, submitted to cranioplasty with custom prosthesis. Figures 5 to 7 illustrate its transoperative period. Originally published in: Prototipagem: aplicações na cirurgia crâniomaxilo-facial do Instituto Nacional de Traumatologia e Ortopedia (INTO)- RJ; Maricevich P, Pantoja E, Mansur A, Peixoto A, Amando J, Borges PYV, André Braune A, Nasser JA, Cruz RL. Revista Brasileira de Cirurgia Plástica 30(4) 2015.
Of the 12 patients who underwent reconstruction with a parietal graft, nine underwent fixation by using RIF 2.0 and three, by using steel wire no. 0 or 1. In patient number 14, we did not use fixation, since bone reconstruction was postponed for a second stage surgery. The time interval between the creation of the defect and its definitive reconstruction ranged from 20 days to 30 years, and patients number 2 and number 16 were submitted to previous attempts at other services (Table 2).
Of the 16 patients who underwent operation, five (30%) evidenced some type of complication. All the complications were resolved without jeopardizing the reconstruction (Table 3).
Patient number 4 presented a lesion of the sagittal sinus during the removal of the parietal graft, which was promptly corrected by using a pericranium patch and hemostatics agents without any hemodynamic repercussion. The same patient evidenced very pronounced system 2.0 plates on the forehead. Because of this irregularity, we decided to re-operate the patient 5 months postoperatively to remove the fixation means and the fat graft.
Patient number 6 evolved with seroma on the 14th postoperative day, and loss of all the HA cement through the wound was evidenced. He underwent conservative treatment by means of serial punctures and digital expression.
In patient number 7, no integration of the parietal graft, which was fixed on the defect, was observed. In the third month of evolution, the graft felt fully mobile and we re-operated the patient to capture more parietal grafts and perform new reconstruction of the defect.
In patient number 11, we observed reduced leakage of CSF while we were dissecting the defect area of a prior trepanation orifice. We corrected the defect by using a pericranium graft with reinforced hemostatic compounds, a biosynthetic dura-mater, and biological glue. Nevertheless, the patient remained in the elevated decubitus position, and we continued the antibiotic therapy and diuretics for 1 week (Figure 11).
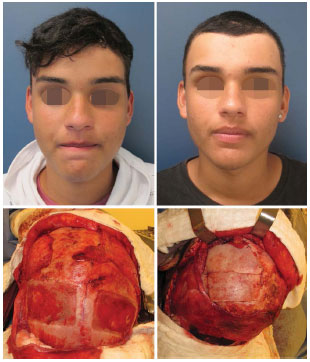
Figure 11. Patient number 11 presented leak of CSF through a prior trepanation during the exposure of the defect. The leak was treated with a pericranium graft, hemostatic, biosynthetic dura-mater, and biological glue. Defect exposed; donor area and pericranium graft; grafts fixed in the defect.
In patient number 13, suffering from a small area of the scalp flap (one digital pulp) was observed. This evolved with partial extrusion of the prosthesis 4 weeks postoperatively. The patient was taken to the surgical center for reoperation, and during the surgery, we decided to retain the prosthesis and covered it with two large patches of the scalp. This decision was based on the absence of any infection and on the appropriate aspect of the prosthesis itself while undergoing an apparent process of integration (Figure 12).
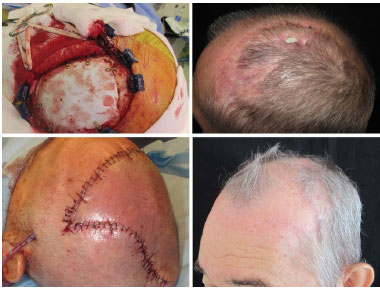
Figure 12. Patient number 13 was submitted to cranioplasty with custom prosthesis because he presented extrusion. The patient was reoperated, while maintaining the same prosthesis. The cover was made with a large rotation flap of the scalp.
In addition, there was a small exposure of the duramater without lesion thereof in patient numbers 6, 7, and 15.
The follow-up of these patients varied from 10 to 22 months. They all, currently, are satisfied with their reconstructions. All the patients underwent reconstruction of the cranial continuity. Various surgical procedures were carried out subsequently to the cranioplasties: in four patients (numbers 2, 3, 4, and 8), a fat graft was used to retouch the contour; in patient number 6, rhinoplasty was performed to correct laterorhinia.
Although we did not quantify it objectively with pre- and postoperative testing, many patients reported improvement in the neurological functions, such as gait and speech. Patient number 1 holds an MSc in Mathematics, is currently finishing his Ph.D., and reported an evident improvement in the concentration during studies.
DISCUSSION
There is no consensus regarding the minimum size of a cranial defect to indicate cranioplasty. Gladstone et al.11 recommended reconstruction of defects of 16 cm2 and Dujovny et al.12, 6 cm2. With regard to skull contour, in addition to the obvious issue of aesthetics, the deformity can often awaken other prejudices with other pathologies in relation to neurological health and the patient's mental capacity6. However, in some patients, skull defects can indeed cause neurological symptoms.
In 1939, Grant and Norcross13 described the syndrome of the trephined in patients with post-craniectomy skull defects. The symptoms include dizziness, fatigue, vague discomfort at the site of the defect, mental depression, anxiety, insecurity, and intolerance to vibration. The pathophysiology of these symptoms is not yet fully clarified, but might be related to changes in the movement of CSF14, or to the effect of the atmospheric pressure by compressing the cortex, or to the reduction of the venous return caused by obliteration of the subarachnoid space15. In 1945, Gardner16 attested the improvement of the neurological function after cranioplasty, which was confirmed later by many other authors 17-24.
In our series, the patients sought medical help mainly to correct the aesthetic deformity and reacquire protection against trauma. Regardless of the size of the defect, the simple psychological impact resulting from constant concern with a possible trauma can already justify a surgical indication, mainly in young active people6. Many of our patients no longer work or practice physical activities because of the cranial defect. All this impairs their social relations and personal aspirations. The report of improvement of some neurological functions in some patients was something that positively surprised us; it represents a benefit that we could not guarantee in the preoperative period (Figures 13 and 14).
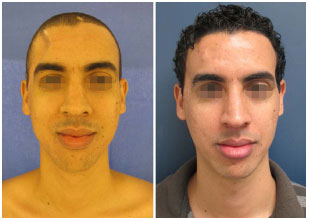
Figure 13. Patient number 1, bearer of an evident cranial defect (sunken skin flap) submitted to parietal cranioplasty. This was one of the patients who, spontaneously, reported improvement in the neurological functions.
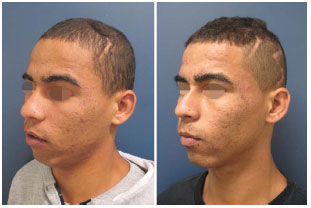
Figure 14. Patient number 12, which was submitted to parietal cranioplasty, also reported improvement in the neurological functions after reconstruction. Figures 2 to 4 illustrate the transoperative period.
Whenever possible, our first choice for cranial reconstructions was the autologous parietal graft. The autologous graft offers lower risk of infection and extrusion compared with the alloplastic materials. However, there is the possibility of absorption; the contour may not be perfect; and there may be a morbidity of the donor area6,25-28. In contrast, a custom prosthesis offers a perfect contour, but there is a higher risk of infection and extrusion25,29-31, and the cost can be a hindrance to its use.
The decision for the reconstruction method in our patients was individualized and passed through several criteria9, but mostly we evaluated the size of the defect and the quality of the donor area by using CT images (quantity and parietal thickness, and definition of the diploe). We used custom prosthesis in two of the patients who evidenced a major defect (192 cm2) and in one patient aged 72 years old. Uygur9 proposed a cranioplasty algorithm in which large defects (> 200 cm2) would be corrected by using either methylmethacrylate, porous polyethylene, or autoclaved dried bone (e.g., in the case of decompressive craniectomies).
The posterior parietal region, 1.5 cm to 2 cm lateral to the sagittal suture, 1 cm posterior to the coronal suture, and 2 cm medial to the squamous suture, was our preference. This region has a thickness of 6.75 mm on average, is thicker in women and in the dark-skinned population; and its thickness does not vary with age in adults32. The greatest difficulty and risk of the parietal graft when harvested in the elderly is related to the absence of a well-defined diploe and increased bone fragility32; as in one of our patients for whom we indicated a prosthesis.
Sustained care during the transoperative period can maximize the viability of the graft. Some examples are: continuous irrigation when using drills and saws, revival of the edges of the defect, maintaining the graft in moist compresses with saline solution for the shortest time possible (maximum 1 hour), exact fit of the graft in the defect, good fixation and stabilization of the graft, filling of the dead spaces with bone material or a pericranium graft, covering the graft with an envelope of thick and well vascularized soft parts, and ensuring that the graft rests on a viable dura-mater10,33,34.
The contact of the graft with the pericranium and dura-mater contributes to the integration of the parietal graft; however, the contact with the dura-mater seems to be more effective and renders the skull a privileged site for large bone grafts35. Therefore, it is important to dissect a defect that is situated on a plane exactly above the duramater, and to leave it clean and in intimate contact with the transplanted bone.
The prostheses used for cranial reconstruction are manufactured using various alloplastic materials, such as HA, titanium, and polymethylmethacrylate. Some materials can be molded during surgery while others are supplied as custom prostheses. These materials must exhibit some characteristics to be considered appropriate: biocompatibility, compatibility with the imaging techniques, ease when handled and shaped, adequate resistance (similar to bone), and be sterilizable.
The two prostheses that we used were from the company Eincobio®; they are customized by using prototyping and are made of porous ceramic (HA and beta tricalcium phosphate). HA displays properties similar to that of the bone (HA constitutes 60% of the bone tissue) and can integrate with the bone tissue. Furthermore, pores of various sizes are important for the migration of both the fibrous tissue and bone tissue inside the prosthesis36.
The results of various experimental studies on animals37 and those obtained after analyzing HA prostheses, which were retrieved in humans38, revealed that the osteoblastic migration takes between four to eight months to complete. It is followed by a good osseointegration in the perimeter of the prosthesis. Following 1 year after the cranioplasty, in some cases of fractures of the prosthesis, there are reports of spontaneous "consolidation" after undergoing conservative treatment36. Patient number 13 evidenced exposure of the HA prosthesis. Furthermore, no signs of infection were present, and we achieved good coverage by using two large rotation flaps of the scalp. However, the main advantage when using the HA prosthesis was its osseointegration; thus, we decided to maintain it in the patient during the reoperation. We believe that another advantage when using a custom HA prosthesis is that it is thicker compared with the titanium one, for example. This means that the dead space between the prosthesis and the dura-mater decreases.
Patient number 14 evidenced ideal conditions to achieve good coverage of the soft parts; thus, we chose not to perform bone reconstruction in this case. Furthermore, when a local infection is present, an immediate cranioplasty is rarely indicated. In such cases, one must wait for a period of 639,40 to 1241-43 months to ensure complete resolution of the infection.
The quality of the coverage of the scalp directly influences the choice of the surgical conduct. Regardless of the material used, cranioplasty must ensure coverage of well vascularized tissue. Local flaps, tissue expansion, and free flaps are alternatives to obtain such a good coverage. Skin grafts are not an option for coverage of cranioplasties.
In large defects or irradiated areas, we must consider free flaps or tissue expansion6. In the case of cranioplasty with autologous bone graft, adding a pericranium graft over the rebuilt area should be performed whenever possible35.
The possible complications of cranioplasties10, mainly when using parietal grafts, are: laceration of the dura-mater, subgaleal hematoma, infection of the surgical site, lack of graft integration, graft absorption, graft or prosthesis extrusion, irregularities in the cranial contour, and possible brain injury44. Lacerations in the dura-mater were avoided by noting the good definition in the diploe of the patient in the preoperative period, and by using good quality surgical material. However, once there was a lesion in the dura-mater, this was considered a mild complication, and we corrected the defect immediately as we would if it were the peritoneum or pleura10.
The lesion of the sagittal sinus in one of our patients was treated in this way without any difficulty. It was shown that the irregularities of the contour do not upset the patient if they are located in hairy areas. However, they may be evident in the frontal region. At this point, a proper fit of the graft and its fixation with low profile RIF (system < 2.0) are important. Unfortunately, due to such system unavailability, in some of our patients we had to use the 2.0 system, although we do not consider it on the forehead. Contour problems were practically inexistent when we used customized prostheses and mini plates.
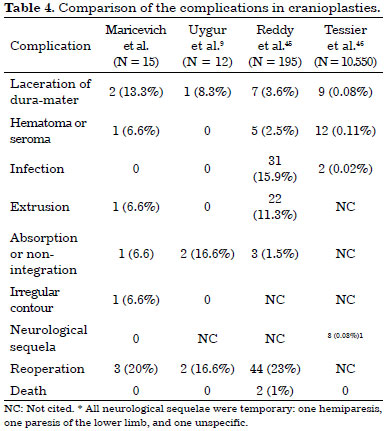
If there are irregularities in the late postoperative period, the solution may be the withdrawal of the fixation, masking it with HA cement and/or fat graft. Our single case of seroma was probably linked to the use of HA cement. We compared our complications of cranial bone reconstruction with those reported in published literature (Table 4).
CONCLUSION
Cranioplasty is a primarily restorative surgery that is used to restore the protective function of the skull and treat the syndrome of the trephined. As a result, it rebuilds the cranial contour, presenting with a large esthetic and resocializing benefit. The autologous parietal graft is the first choice whenever possible. The alloplastic prosthesis is indicated mainly when there is a large defect or when the harvest of parietal graft is not possible for some reason: inadequate thickness or poorly defined diploe.
To perform a cranial reconstruction with a parietal graft, it is fundamental to use surgical material of good quality and to have knowledge of the anatomy, while employing CT analysis. When the choice is for the custom prosthesis, the cranial contour is a perfect choice.
ACKNOWLEDGMENTS
I want to thank Dr. José Augusto Nasser, neurosurgeon, who provided support in all these neurosurgical operations. Moreover, I want to thank Dr Ricardo Cruz, head of the CAE-CMF of the INTO-RJ, my professor and mentor in craniofacial surgery, who besides the theoretical and practical aspects of the surgery, exemplifies in everyday life, the holistic and human vision that we should have of our patients. In addition, I thank the entire multidisciplinary team of the INTO -RJ, where I learned much more than just to place plates and screws.
REFERENCES
1. Hara T, Farias CASA, Costa MJM, Cruz RJL. Cranioplastia: parietal versus prótese customizada. Rev Bras Cir Plást. 2011;26(1):32-6. DOI: http://dx.doi.org/10.1590/S1983-51752011000100008
2. Agrawal A, Garg LN. Split calvarial bone graft for the reconstruction of skull defects. J Surg Tech Case Rep. 2011;3(1):13-6. DOI: http://dx.doi.org/10.4103/2006-8808.78465
3. Artico M, Ferrante L, Pastore FS, Ramundo EO, Cantarelli D, Scopelliti D, et al. Bone autografting of the calvaria and craniofacial skeleton: historical background, surgical results in a series of 15 patients, and review of the literature. Surg Neurol. 2003;60(1):71-9. DOI: http://dx.doi.org/10.1016/S0090-3019(03)00031-4
4. Neligan PC, Boyd JB. Reconstruction of the cranial base defect. Clin Plast Surg. 1995;22(1):71-7.
5. Erculei F, Walker AE. Posttraumatic epilepsy and early cranioplasty. J Neurosurg. 1963;20:1085-9. PMID: 14186110 DOI: http://dx.doi.org/10.3171/jns.1963.20.12.1085
6. Baumeister S, Peek A, Friedman A, Levin LS, Marcus JR. Management of postneurosurgical bone flap loss caused by infection. Plast Reconstr Surg. 2008;122(6):195e-208e. PMID: 19050490 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181858eee
7. Psillakis JM, Nocchi VL, Zanini SA. Repair of large defect of frontal bone with free graft of outer table of parietal bones. Plast Reconstr Surg. 1979;64(6):827-30. PMID: 390579 DOI: http://dx.doi.org/10.1097/00006534-197912000-00023
8. Tessier P. Autogenous bone grafts taken from the calvarium for facial and cranial applications. Clin Plast Surg. 1982;9(4):531-8.
9. Uygur S, Eryilmaz T, Cukurluoglu O, Ozmen S, Yavuzer R. Management of cranial bone defects: a reconstructive algorithm according to defect size. J Craniofac Surg. 2013;24(5):1606-9. DOI: http://dx.doi.org/10.1097/SCS.0b013e3182a2101c
10. Tessier P, Kawamoto H, Posnick J, Raulo Y, Tulasne JF, Wolfe SA. Taking calvarial grafts, either split in situ or splitting of the parietal bone flap ex vivo--tools and techniques: V. A 9650-case experience in craniofacial and maxillofacial surgery. Plast Reconstr Surg. 2005;116(5 Suppl):54S-71S. PMID: 16217445 DOI: http://dx.doi.org/10.1097/01.prs.0000173949.51391.d4
11. Gladstone HB, McDermott MW, Cooke DD. Implants for cranioplasty. Otolaryngol Clin North Am. 1995;28(2):381-400.
12. Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: cosmetic or therapeutic? Surg Neurol. 1997;47(3):238-41. PMID: 9068693
13. Grant FC, Norcross NC. Repair of cranial defect by cranioplasty Ann Surg. 1939;110(4):488-512.
14. Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res. 1997;19(3):311-6.
15. Richaud J, Boetto S, Guell A, Lazorthes Y. Effects of cranioplasty on neurological function and cerebral blood flow. Neurochirurgie. 1985;31(3):183-8.
16. Gardner WJ. Closure of defects of the skull with tantalum. Surg Gynecol Obstet. 1945;80:303-12.
17. Grantham EC, Landis HP. Cranioplasty and the post-traumatic syndrome. J Neurosurg. 1948;5(1):19-22.
18. Agner C, Dujovny M, Gaviria M. Neurocognitive assessment before and after cranioplasty. Acta Neurochir (Wien). 2002;144(10):1033-40.
19. Harner SG, Beatty CW, Ebersold MJ. Impact of cranioplasty on headache after acoustic neuroma removal. Neurosurgery. 1995;36(6):1097-9.
20. Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53(3):288-92.
21. Schiffer J, Gur R, Nisim U, Pollak L. Symptomatic patients after craniectomy. Surg Neurol. 1997;47(3):231-7. PMID: 9068692
22. Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery. 1994;34(4):729-31.
23. Tabaddor K, LaMorgese J. Complication of a large cranial defect. Case report. J Neurosurg. 1976;44(4):506-8. PMID: 1255240
24. Yamaura A, Sato M, Meguro K, Nakamura T, Uemura K. Cranioplasty following decompressive craniectomy--analysis of 300 cases (author's transl). No Shinkei Geka. 1977;5(4):345-53.
25. Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD, et al. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: a systematic review. Neurosurgery. 2011;68(4):1124-9.
26. Cheng YK, Weng HH, Yang JT, Lee MH, Wang TC, Chang CN. Factors affecting graft infection after cranioplasty. J Clin Neurosci. 2008;15(10):1115-9.
28. Gao LL, Rogers GF, Clune JE, Proctor MR, Meara JG, Mulliken JB, et al. Autologous cranial particulate bone grafting reduces the frequency of osseous defects after cranial expansion. J Craniofac Surg. 2010;21(2):318-22.
29. Sahoo N, Roy ID, Desai AP, Gupta V. Comparative evaluation of autogenous calvarial bone graft and alloplastic materials for secondary reconstruction of cranial defects. J Craniofac Surg. 2010;21(1):79-82.
30. Gosain AK; Plastic Surgery Eductional Foundation DATA Committee. Biomaterials for reconstruction of the cranial vault. Plast Reconstr Surg. 2005;116(2):663-6. PMID: 16079708
31. Zins JE, Langevin CJ, Nasir S. Controversies in skull reconstruction. J Craniofac Surg. 2010;21(6):1755-60. DOI: http://dx.doi.org/10.1097/SCS.0b013e3181c34675
32. Moreira-Gonzalez A, Papay FE, Zins JE. Calvarial thickness and its relation to cranial bone harvest. Plast Reconstr Surg. 2006;117(6):1964-71. PMID: 16651971 DOI: http://dx.doi.org/10.1097/01.prs.0000209933.78532.a7
33. Park HK, Dujovny M, Agner C, Diaz FG. Biomechanical properties of calvarium prosthesis. Neurol Res. 2001;23(2-3):267-76. DOI: http://dx.doi.org/10.1179/016164101101198424
34. Netscher DT, Stal S, Shenaq S. Management of residual cranial vault deformities. Clin Plast Surg. 1992;19(1):301-13. PMID: 1537225
35. Sweeney W, Gosain SA, Santoro TD, Song L, Amarante MT, Gosain AK. What makes the calvaria a privileged site for bone graft survival? Plast Reconstr Surg. 2010;126(Suppl 4S):2. DOI: http://dx.doi.org/10.1097/01.prs.0000388719.39193.c0
36. Stefini R, Esposito G, Zanotti B, Iaccarino C, Fontanella MM, Servadei F. Use of "custom made" porous hydroxyapatite implants for cranioplasty: postoperative analysis of complications in 1549 patients. Surg Neurol Int. 2013;4:12. DOI: http://dx.doi.org/10.4103/2152-7806.106290
37. Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49(3):328-37. DOI: http://dx.doi.org/10.1002/(SICI)1097-4636(20000305)49:3<328::AID-JBM5>3.0.CO;2-Q
38. Messina G, Dones I, Nataloni A, Franzini A. Histologically demonstrated skull bone integration in a hydroxyapatite prosthesis in a human. Acta Neurochir (Wien). 2011;153(8):1717-8. DOI: http://dx.doi.org/10.1007/s00701-011-1014-5
39. Lee C, Antonyshyn OM, Forrest CR. Cranioplasty: indications, technique, and early results of autogenous split skull cranial vault reconstruction. J Craniomaxillofac Surg. 1995;23(3):133-42. DOI: http://dx.doi.org/10.1016/S1010-5182(05)80001-0
40. Eppley BL, Kilgo M, Coleman JJ 3rd. Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: indications, surgical technique, and long-term follow-up. Plast Reconstr Surg. 2002;109(3):864-71. DOI: http://dx.doi.org/10.1097/00006534-200203000-00005
41. Manson PN, Crawley WA, Hoopes JE. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986;77(6):888-904. PMID: 3520618 DOI: http://dx.doi.org/10.1097/00006534-198606000-00003
42. Hammon WM, Kempe LG. Methyl methacrylate cranioplasty. 13 years experience with 417 patients. Acta Neurochir (Wien). 1971;25(1):69-77.
43. Rish BL, Dillon JD, Meirowsky AM, Caveness WF, Mohr JP, Kistler JP, et al. Cranioplasty: a review of 1030 cases of penetrating head injury. Neurosurgery. 1979;4(5):381-5. PMID: 111153 DOI: http://dx.doi.org/10.1227/00006123-197905000-00002
44. Kline RM Jr, Wolfe SA. Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg. 1995;95(1):5-13. DOI: http://dx.doi.org/10.1097/00006534-199501000-00002
45. Reddy S, Khalifian S, Flores JM, Bellamy J, Manson PN, Rodriguez ED, et al. Clinical outcomes in cranioplasty: risk factors and choice of reconstructive material. Plast Reconstr Surg. 2014;133(4):864-73. PMID: 24675189 DOI: http://dx.doi.org/10.1097/PRS.0000000000000013
46. Tessier P, Kawamoto H, Posnick J, Raulo Y, Tulasne JF, Wolfe SA. Complications of harvesting autogenous bone grafts: a group experience of 20,000 cases. Plast Reconstr Surg. 2005;116(5 Suppl):72S-73S. PMID: 16217446 DOI: http://dx.doi.org/10.1097/01.prs.0000173841.59063.7e
1. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
2. Associação Brasileira de Cirurgia Crânio-Maxilo-Facial, São Paulo, SP, Brazil
3. Instituto Nacional de Traumatologia e Ortopedia, Rio de Janeiro, RJ, Brazil
4. Sociedade Brasileira de Neurocirurgia, São Paulo, SP, Brazil
5. Academia Nacional de Medicina, Rio de Janeiro, RJ, Brazil
Institution: Instituto Nacional de Traumatologia e Ortopedia - RJ Clínica Privada.
Corresponding author:
Pablo Maricevich
Av. Antônio de Góes, 275, Pina
Recife, PE, Brazil Zip Code 51110-000
E-mail: jpmaricevich@hotmail.com
Article received: October 12, 2014.
Article accepted: April 21, 2015.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter