

Original Article - Year 2016 - Volume 31 -
Galactorrhea: how to address this unusual complication after augmentation mammoplasty
Galactorreia: como abordar essa complicação incomum após mamoplastia de aumento
ABSTRACT
INTRODUCTION: Galactorrhea and galactocele formation after breast augmentation are complications reported in the literature, but the cause remains unknown.
METHODS: We present a case of a 28-year-old patient who underwent breast augmentation surgery via the inframammary fold with an implant placed in the subfascial plane, which developed galactorrhea from the incision on the seventh postoperative day, and we propose an algorithm for the diagnosis and treatment of galactorrhea after mammoplasties.
RESULTS: The complication was treated with the use of a lactation suppressor, cabergoline, presenting good outcomes.
CONCLUSION: Galactorrhea is an uncommon complication after augmentation mammoplasties, which should always be considered in cases of secretions from an incision because it is a differential diagnosis for infections.
Keywords: Galactorrhea; Mammoplasty; Breast implant; Ergolines.
RESUMO
INTRODUÇÃO: Galactorreia e formação de galactocele após mamoplastia de aumento é uma complicação descrita na literatura, porém a causa permanece desconhecida.
MÉTODOS: Apresentamos um caso de uma paciente de 28 anos que foi submetida à cirurgia de mamoplastia de aumento via sulco inframamário, com implante colocado no plano subfascial, que evoluiu, no 7º dia pós-operatório, com galactorreia exteriorizada pela incisão, e propomos um algoritmo para diagnóstico e tratamento de galactorreia após mamoplastias.
RESULTADOS: A complicação foi tratada com o uso de agente supressor da lactação, a cabergolina, apresentando boa evolução.
CONCLUSÃO: Galactorreia é uma complicação incomum após mamoplastias de aumento, que deve ser sempre lembrada em casos de drenagem de secreção pela incisão por tratar-se de um diagnóstico diferencial com infecção.
Palavras-chave: Galactorreia; Mamoplastia; Implante mamário; Ergolinas.
Silicone breast implants have been used for more than five decades for breast augmentation for aesthetic purposes, as well as breast reconstruction after mastectomy. Breast augmentation is one of the most frequently performed surgeries worldwide. The use of silicone breast implants, similar to any other surgical procedures, is associated with complications. The majority of these are related to the surgery and can be reduced with an appropriate surgical technique1.
In the immediate postoperative period, hematoma has been reported as the most common complication, with incidence rates of 0.5-3%2.
The indiscriminate use of electrocautery may promote seroma formation. Seroma is usually absorbed in 4-5 weeks, but drainage can be performed under the guidance of ultrasound or by endoscopic surgery if indicated3.
Infection, although rare, occurs at a rate consistent with the expected rate after any other surgery with prosthetic implantation. The incidence is related to the procedure (higher in breast reconstruction than in breast augmentation) and has been reported in several studies with a rate ranging from 1% to 7%1,4. A statistically significant evidence was found in favor of antibiotic prophylaxis, but no consensus is obtained regarding its use4,5. Staphylococci are the most common microorganisms6. Rare pathologic agents include atypical mycobacteria and fungi7.
A review of the literature shows few reports of patients with galactocele and/or galactorrhea after breast augmentation8-10. Although an uncommon complication, it must always be considered in cases of secretions from an incision because it is a differential diagnosis for infections.
OBJECTIVE
This article describes an unusual complication of breast augmentation, galactorrhea, and proposes an algorithm for the management of this complication.
METHODS
We present a case of a 28-year-old patient who underwent breast augmentation via the inframammary fold with a round, textured, 255 cc implant, placed in the subfascial plane. The surgery did not present intercurrences, and lasted approximately 75 min. She was discharged on the first postoperative day with analgesics and cefalexin.
The patient did not have a history of comorbidities or medication use. She had a ten-year-old son, who was born by normal delivery. She used oral contraceptives at the time of surgery.
She presented with small amounts of serous discharge from the surgical wound in the first three days. On the seventh postoperative day, a whitish, milky secretion was observed (Figure 1), without inflammation signs, from the incision of the left breast. A sample of the secretion was harvested and cultured, and ciprofloxacin therapy and reassessment every two days were chosen. Her secretions continued in moderate amounts in subsequent days, despite antibiotic therapy. The beginning of secretion with the same characteristics in the right breast was also observed. The culture was negative for common bacteria. The patient was stable, without systemic signs of infection and inflammation signs in the breasts.
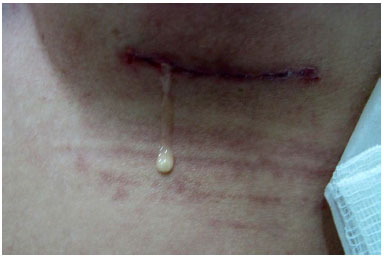
Figure 1. Detailed appearance of the surgical wound secretion.
Mycobacterium infection was suspected, and the secretions were collected for culture of atypical mycobacteria and fungi. All cultures were negative.
On the 20th postoperative day, nipple secretion continued, with galactorrhea observed bilaterally in the lactiferous ducts, whereas the secretion exuded by the surgical wound presented the same physical characteristics of the nipple secretion (Figure 2)
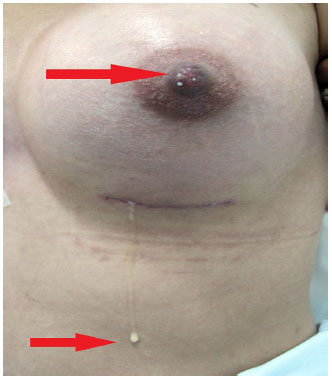
Figure 2. Milky secretion from the surgical wound and the nipple.
Two tablets of 0.5-mg cabergoline were orally administered. The patient improved, and secretions stopped 7 days after cabergoline use, presenting symmetric breasts and satisfaction with the outcome (Figure 3). Montelukast, a leukotriene inhibitor, 10 mg/day, was prescribed for 3 months.
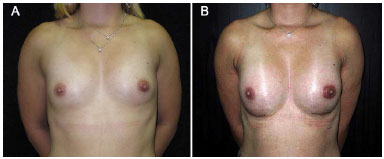
Figure 3. A: preoperative photo; B: postoperative photo, after treatment with cabergoline.
RESULTS
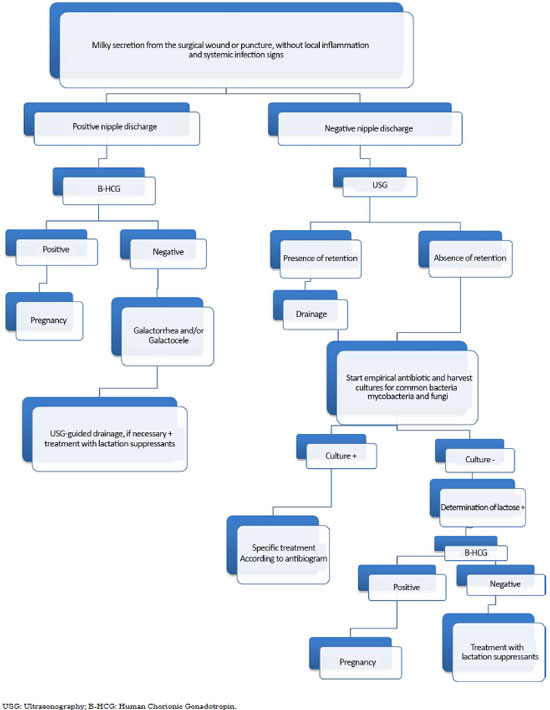
We propose an algorithm for the diagnosis and treatment of this rare complication to facilitate its management (Figure 4).
For patients in the postoperative period of a recent breast augmentation, who present with whitish, milky secretion exuding from the surgical wound or from a puncture, and who do not present signs of inflammation in the breasts or systemic signs of infection, such as fever, malaise and prostration, the presence of secretions from the nipples should be checked to evaluate the presence of galactorrhea from the lactiferous ducts.
If nipple discharge is positive, the secretion from the surgical wound will also probably be galactorrhea. Endocrine and human chorionic gonadotropin (B-HCG) level evaluations are recommended to rule out pituitary changes and pregnancy, respectively. If B-HCG is negative, ultrasonography and guided drainage are performed in cases of milk retention (galactocele), which is associated with the systemic use of lactation suppressors.
Determination of lactose in the secretion can be used to confirm the diagnosis of galactorrhea/galactocele. In cases wherein nipple discharge is negative, the algorithm is performing an ultrasound to rule out retentions, collecting secretions for common bacteria cultures, such as mycobacteria and fungi, and administering antibiotic therapy empirically until culture results are obtained. If the culture is positive, specific treatment is administered based on the antibiogram. If the culture is negative, the determination of lactose in the secretion is recommended to confirm galactorrhea and its treatment with lactation suppressors and drainage, if necessary.
Cabergoline is an ergot dopaminergic derivative, which is a potent and prolonged suppressant of prolactin. The recommended dose is 0.5 mg per week, administered in 1 or 2 doses.
Because no data exist regarding the index of capsular contracture in these patients who present with galactorrhea or galactocele, we speculate that using leukotriene inhibitors, in these patients, for 3 months is sensible.
DISCUSSION
Augmentation mammoplasty does not alter the lactating function of the breast. The majority of patients can breastfeed after surgery11-13. Hurst14 reported that the periareolar approach presented a higher complication rate associated with lactation.
Galactorrhea and formation of galactocele, requiring drainage and use of lactation suppressants, have been reported. The cause and incidence rate of these events are unknown10,15,16. Lactation can begin a few days after mammoplasty10,17.
Acartürk et al.9 reported a case of a patient with galactocele in both breasts 2 years after periareolar, transglandular, and subpectoral augmentation; however, the patient was in the last month of pregnancy.
Deloach et al.10 also reported two cases of patients with unilateral galactocele after breast augmentation. In the first case, the implant was removed one month after surgery, and the patient underwent breast augmentation for the second time. In the second case, the patient underwent exploratory surgery, wherein the milky secretion was drained, and the same implant was placed again. Based on the author, galactocele formation may be attributable to the handling of the breast tissue during surgery. An incision in the inframammary region is preferred by some surgeons to minimize the risk of disturbance to the lactiferous ducts. The use of oral contraceptives can also have a role in this process10.
Hugill18 reported that lactation may be interrupted spontaneously, without medication.
Rothkopf and Rosen19 successfully treated lactation after mammoplasty with bromocriptine. In some cases, the rapid resolution of lactation can be achieved with the use of bromocriptine, suppressing the high prolactin level10,19. However, bromocriptine is not currently indicated as the best treatment for the prevention of physiological lactation because it increases the risk of cerebral vascular accident and seizures in the postpartum period20.
Galactorrhea is an uncommon complication after augmentation mammoplasties, which should be considered by plastic surgeons in cases of milky secretion discharged from the incision because it is a differential diagnosis for infections to prevent precipitated implant withdrawal. Most of the studies in the literature report drainage as the approach to galactorrhea and galactocele, when necessary, and the use of lactation suppressants.
CONCLUSION
The cause of galactocele and galactorrhea after augmentation mammoplasties is unclear. It is hypothesized that the manipulation of the breast tissue, with lesion of the lactiferous ducts during surgery, is related with its genesis. However, in the case presented here, the patient had no damaged glandular breast tissue because the implants were placed in the inframammary fold and subfascial plane. We expect that this documentation will provide a basis for future research, and that the proposed algorithm will be useful in the management of this complication.
COLLABORATIONS
ASKA Statistical analysis; conception and design of the study; completion of surgeries and/or experiments; and writing the manuscript or critical review of its contents.
RG Analysis and/or interpretation of data; final approval of the manuscript.
IMJ Completion of surgeries and/or experiments; writing of the manuscript or critical review of its contents.
PB Completion of surgeries and/or experiments.
RSF Analysis and/or interpretation of data; final approval of the manuscript.
REFERENCES
1. Iwuagwu FC, Frame JD. Silicone breast implants: complications. Br J Plast Surg. 1997;50(8):632-6. DOI: http://dx.doi.org/10.1016/S0007-1226(97)90509-9
2. Baker JL. Augmentation mammaplasty. In: Owsley JQ Jr, Peterson RA, eds. Symposium on Aesthetic Surgery of the Breast. St Louis: Mosby; 1978. p.25-63.
3. Shafir R, Heyman Z, Tsur H, Itzchak Y. Ultrasound scanning as an aid in the diagnosis and treatment of periprosthetic hematoma after breast surgery. Plast Reconstr Surg. 1983;71(6):858-60. PMID: 6856702 DOI: http://dx.doi.org/10.1097/00006534-198306000-00023
4. Clegg HW, Bertagnol l P, Hightower AW, Baine WB. Mammaplasty-associated mycobacterial infection: a survey of plastic surgeons. Plast Reconstr Surg. 1983;72(2):165-9. PMID: 6878490 DOI: http://dx.doi.org/10.1097/00006534-198308000-00007
5. Brand KG. Infection of mammary prostheses: a survey and the question of prevention. Ann Plast Surg. 1993;30(4):289-95. DOI: http://dx.doi.org/10.1097/00000637-199304000-00001
6. McGrath MH, Burkhardt BR. The safety and efficacy of breast implants for augmentation mammaplasty. Plast Reconstr Surg. 1984;74(4):550-60. PMID: 6385039 DOI: http://dx.doi.org/10.1097/00006534-198410000-00019
7. Williams K, Walton RL, Bunkis J. Aspergillus colonization associated with bilateral silicone mammary implants. Plast Reconstr Surg. 1983;71(2):260-1. PMID: 6823489 DOI: http://dx.doi.org/10.1097/00006534-198302000-00024
8. Caputy GG, Flowers RS. Copious lactation following augmentation mammaplasty: an uncommon but not rare condition. Aesthetic Plast Surg. 1994;18(4):393-7. PMID: 7817889 DOI: http://dx.doi.org/10.1007/BF00451346
9. Acartürk S, Gencel E, Tuncer I. An uncommon complication of secondary augmentation mammoplasty: bilaterally massive engorgement of breasts after pregnancy attributable to postinfection and blockage of mammary ducts. Aesthetic Plast Surg. 2005;29(4):274-9. DOI: http://dx.doi.org/10.1007/s00266-005-1093-x
10. Deloach ED, Lord SA, Ruf LE. Unilateral galactocele following augmentation mammoplasty. Ann Plast Surg. 1994;33(1):68-71. PMID: 7944201 DOI: http://dx.doi.org/10.1097/00000637-199407000-00013
11. Grant S, Edelman DA. Pregnancy, lactation and the use of silicone breast implants. Adv Contracept. 1994;10(3):187-93. PMID: 7863844 DOI: http://dx.doi.org/10.1007/BF01983350
12. Lewis JR. Augmentation mammoplasty. In: Georgiade NG, ed. Aesthetic breast surgery (Chap. 4). Baltimore: Williams and Wilkins; 1983. p.24-49.
13. Riefkohl R. Augmentation mammoplasty. In: McCarthy JG, May JW Jr, Littler JW, eds. Plastic Surgery. Saunders: Philadelphia; 1990. p.3879-92. DOI: http://dx.doi.org/10.2134/jeq1990.192349x
14. Hurst NM. Lactation after augmentation mammoplasty. Obstet Gynecol. 1996;87(1):30-4. PMID: 8532261 DOI: http://dx.doi.org/10.1016/0029-7844(95)00349-5
15. Hartley JH Jr, Schatten WE. Postoperative complication of lactation after augmentation mammaplasty. Plast Reconstr Surg. 1971;47(2):150-3. PMID: 5107582 DOI: http://dx.doi.org/10.1097/00006534-197102000-00009
16. Silverman BG, Brown SL, Bright RA, Kaczmarek RG, Arrowsmith-Lowe JB, Kessler DA. Reported complications of silicone gel breast implants: an epidemiologic review. Ann Intern Med. 1996;124(8):744-56. DOI: http://dx.doi.org/10.7326/0003-4819-124-8-199604150-00008
17. Hartley JH Jr, Schatten WE. Postoperative complication of lactation after augmentation mammaplasty. Plast Reconstr Surg. 1971;47(2):150-3. PMID: 5107582 DOI: http://dx.doi.org/10.1097/00006534-197102000-00009
18. Hugill JV. Lactation following breast augmentation: a third case. Plast Reconstr Surg. 1991;87(4):806-7. PMID: 2008487 DOI: http://dx.doi.org/10.1097/00006534-199104000-00041
19. Rothkopf DM, Rosen HM. Lactation as a complication of aesthetic breast surgery successfully treated with bromocriptine. Br J Plast Surg. 1990;43(3):373-5. DOI: http://dx.doi.org/10.1016/0007-1226(90)90095-H
20. Jacob LS. Hormones, antagonists, and other agents affecting endocrine function: IV. Diabetes mellitus and insulin therapy. In: Jacob LS. Pharmocology. Philadelphia: Williams & Wilkins; 1996. p. 219-47.
1. Hospital de Clínicas, Universidade Federal do Paraná, Curitiba, PR, Brazil
2. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
Institution: Hospital de Clínicas de Curitiba, Universidade Federal do Paraná, Curitiba, PR, Brazil.
Corresponding author:
Adriana Sayuri Kurogi Ascenço
Rua Solimões, 1175 - Bairro Mercês
Curitiba, PR, Brazil Zip Code 80810-070
E-mail: sayurikurogi@hotmail.com
Article received: July 20, 2012.
Article accepted: May 2, 2016.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter