
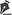
Case Reports - Year 2016 - Volume 31 -
Late complication of cutaneous filling after a facelift: a case report
Complicação tardia de preenchimento cutâneo após facelift: relato de caso
ABSTRACT
The authors present the case of a patient who underwent a facelift with a chin implant 7 years after polymethylmethacrylate (PMMA) implantation, which evolved with foreign body granuloma in a region distant from the filling application. After nearly a year of treatment, the patient evolved with resolution of the granuloma.
Keywords: Foreign body granuloma; PMMA; Facelift/adverse effects
RESUMO
Os autores apresentam o caso de uma paciente submetida 7 anos após aplicação de polimetilmetacrilato (PMMA) a um facelift com implante de prótese mentoniana, o qual evoluiu com granuloma por corpo estranho em região distante da aplicação do preenchimento. Após quase um ano de tratamento, a paciente evoluiu com resolução do caso.
Palavras-chave: Granuloma de corpo estranho; PMMA; Erguimento da face/efeitos adversos.
The aging process causes a decrease in subcutaneous fat and dermal collagen, resulting in grooves and facial depressions that are accentuated over time owing to volumetric loss and muscle action. Treatment of facial aging, previously focused only on the traction of the tissues, is currently based on restoration of volume and facial contours.
The ideal filler must be autologous, long-lasting, or permanent without immunological or toxic effects and should act to slow down or prevent the aging process. Permanent fillers such as paraffin, liquid silicone, Teflon, and polymethylmethacrylate (PMMA) have a rough surface that cannot be phagocytosed, eventually forming granulomas.
Particles smaller than 15 µm are generally phagocytized and can be transported to lymph nodes. From a clinical perspective, all injectable fluids cause foreign-body reaction to varying degrees. Until the mechanism of granuloma formation is fully understood, it will not be possible to predict its development1,2.
CASE REPORT
A 53-year-old white woman who had no comorbidities or smoking habit underwent PMMA filling in the nasolabial folds, which was performed by a colleague, 7 years before a facelift. On June 7, 2011, she underwent a facelift and chin silicone prosthesis implant without intercurrences and developed a hematoma in the left hemiface in the immediate postoperative period, which was immediately drained. On the 30th postoperative day, 2 ml of seroma was aspirated from the left submandibular region, with new drainage being performed 20 days after the first.
On September 12 and 19, infiltration with dipropionate and betamethasone phosphate was performed; but on September 25, an organized tumorous formation was observed in the left submandibular region. Ultrasonography was performed in this region and indicated evidence of a solid, heterogeneous nodular formation, with interposed echogenic foci and Doppler flow, measuring 2.6 x 1.3 cm, besides two well-defined hypoechoic formations, measuring 1.3 x 0.7 and 1.5 x 0.7 cm, respectively, adjacent to the main lesion. No alterations in the submandibular glands nor lymphadenopathy were observed.
On October 5, nuclear magnetic resonance imaging was performed and revealed lymphadenopathy in the left submandibular region, bilateral volumetric increase of the tonsils, lymphadenomegaly to the right, and ovoid subcutaneous images with low signal intensities on T1 and T2 in the submandibular area due to the cutaneous filling material.
On October 19 (Figure 1), biopsies of the lesions were performed, which resulted in a nonspecific chronic inflammatory process with erosion in the samples. On October 28, the patient underwent excision of the lesions (Figure 2), and a pathological study result showed a foreign body-type granulomatous process in all the samples.
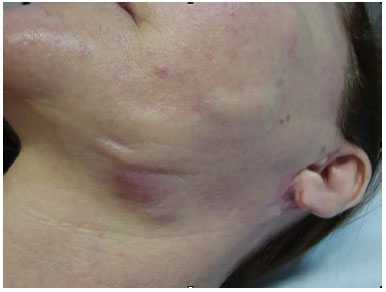
Figure 1. Skin lesions with local inflammatory reaction.
Figure 2. Aspect of the lesion during the excision.
Twenty-five days after resection of the lesion, the patient evolved with a new inflammatory process, this time in the right submandibular region. Thus, administration of 500 mg of ciprofloxacin every 12 hours was initiated, which resulted in a significant improvement of the lesions. New infiltration with betamethasone was performed on January 30, 2012. On May 9, 2012, the lesions were excised, thereby achieving resolution of the case.
DISCUSSION
Facial aging is characterized basically by flaccidity of tissues, formation of wrinkles, and volumetric reduction of the face with variable intensity. This flaccidity can be corrected by several surgical techniques, but these techniques are all inadequate for correcting the loss of subcutaneous volume and leave marks on the skin via the muscular action during aging.
A more ideal solution would be fat grafting, but the lack of uniformity of results, difficulty of graft vascularization, fat absorption, and the need for surgical harvesting procedures have led to the search for an appropriate fat replacement3.
In approaching a patient who desires esthetic improvement of these areas, the physician should be able to assess the quality and position of the subcutaneous tissues. With an assessment of facial esthetics and a practical knowledge of the capabilities and limitations of each filler, the plastic surgeon will be able to use the most appropriate filler to achieve better esthetic results with the lowest risk to the patient4.
The most recent use of PMMA dates back to when a concern emerged in the past regarding the use of liquid silicone5. PMMA, due to its low cost and unrestricted control of sales, is being used indiscriminately by nonmedical professionals and physicians without appropriate expertise. In addition, granulomatous complications may occur regardless of the technique used6.
PMMA is non-allergenic but depends on the vehicle used to be considered completely inert in the body. Complications can appear in the form of granuloma, when its application is superficial. The use of microcannulas prevents ecchymosis and intravascular injections3.
Regarding histological changes, PMMA implantation in the body induces the production of macrophages, which indicates microspheres of incompatible sizes for phagocytosis and the process evolves to granuloma formation3,6. According to the literature, granuloma formation occurs as a complication at a rate of 0.01% to 2.5% of all PMMA applications, considering the use of PMMA fillers from different manufacturers. The presence of small nodules and painless masses is common after the application of PMMA but is almost never a formal complaint of patients.
Lemperle et al.7 described three mechanisms by which PMMA microspheres can migrate, as follows: the hematogenic route by inadvertent intravascular injection; the lymphatic route by accidental injection of thick lymphatic vessels, with the local lymph nodes and lungs as the more probable final destinations; and phagocytosis of microspheres by macrophages in the implantation site, which then migrate to local lymph nodes.
Neves et al.8 performed an experimental study in rats after intradermal infiltration of PMMA and in the glabella. After 30 days, the animals were killed and samples of the lungs, liver, spleen, and kidneys were submitted for histopathological study. No PMMA microspheres were found in the tissues analyzed.
The reported case refers to that of a 53-year-old patient who was previously subjected to PMMA filling in the nasolabial folds and submitted to a facelift and chin prosthesis implant after 7 years, evolving with foreign-body granuloma in a location distant from the site of PMMA application. As no lymph node was found in the anatomopathological study of the lesions, despite being observed on the nuclear magnetic resonance imaging requested, the migration path of the alloplastic materials in this case is, therefore, obscure.
CONCLUSION
Permanent fillers should be used with caution. Clinical treatment is the first option for complications and the surgical treatment of selected cases in which the clinical treatment was not successful6, as in our case. The treatment is long and complex, requiring caution on the part of the surgeon and perseverance of the patient.
COLLABORATIONS
RNS Analysis and/or data interpretation; statistical analysis; conception and design of the study; completion of operations and/or experiments; and writing of the manuscript or critical review of its contents
SGM Analysis and/or data interpretation; statistical analysis; conception and design of the study; completion of operations and/or experiments; and writing of the manuscript or critical review of its contents
ECA Analysis and/or data interpretation; statistical analysis; and writing of the manuscript or critical review of its contents
ALAF Analysis and/or data interpretation; statistical analysis; and writing of the manuscript or critical review of its contents
ÊGA Analysis and/or data interpretation; statistical analysis; and writing of the manuscript or critical review of its contents
LASL Final approval of the manuscript
REFERENCES
1. Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 Suppl):7S-14S. PMID: 16936539 DOI: http://dx.doi.org/10.1097/01.prs.0000234900.26676.0b
2. Athre RS. Facial filler agents. Op Tech Otolaryngol. 2007;18(3):243-7. DOI: http://dx.doi.org/10.1016/j.otot.2007.07.002
3. Passy S. Procedimentos estéticos ancilares: Parte II Metacrill. In: Thomas RL, Badin AZD, Moraes LM, eds. Rejuvenescimento facial: cirurgia videoendoscópica e procedimentos ancilares. Rio de Janeiro: Revinter; 2003. p.280-91.
4. Tan SR, Glogau RG. A estética dos preenchedores. In: Carruthers J, Carruthers A, eds. Técnicas de preenchimentos. Série procedimentos em dermatologia cosmética. Rio de Janeiro: Elsevier; 2005. p.11-9.
5. Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006;118(3 Suppl):77S-84S. PMID: 16936547 DOI: http://dx.doi.org/10.1097/01.prs.0000234919.25096.67
6. Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123(6):1842-63. PMID: 19483587 DOI: http://dx.doi.org/10.1097/PRS.0b013e31818236d7
7. Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL. Migration studies and histology of injectable microspheres of different sizes in mice. Plast Reconstr Surg. 2004;113(5):1380-90. PMID: 15060350 DOI: http://dx.doi.org/10.1097/01.PRS.0000112764.22839.7A
8. Neves Rd, Herdt MA, Wohlgemuth FB, Ely JB, de Vasconcellos ZA, Bastos JC, et al. Possible migration and histopathological analysis of injections of polymethylmethacrylate in wistar rats. ISRN Dermatol. 2012;2012:609158. PMID: 22701181
1. Hospital Agamenon Magalhães, Recife, PE, Brazil
2. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
Institution: Hospital Agamenon Magalhães, Recife, PE, Brazil.
Corresponding author:
Rafael Neves de Souza
Av. Visconde de Jequitinhonha, 1144, sala 606 - Boa Viagem
Recife, PE, Brazil Zip Code 51030-020
E-mail: raf.nev@uol.com.br
Article received: March 3, 2013.
Article accepted: July 20, 2013.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter