

Original Article - Year 2016 - Volume 31 -
Subcicatricial fat grafting associated with rigotomy: a clinical and microstructural analysis
Lipoenxertia subcicatricial associada à rigottomy: uma análise clínica e microestrutural
ABSTRACT
INTRODUCTION: The increase in survival of large burn patients during the acute phase has also increased the prevalence of esthetic-functional scarring sequelae. With regard to the clinical relevance of subcicatricial fat grafting, the actual changes after the procedure need to be understood and microscopically evaluated.
METHODS: Eight patients with alcohol burns, with an average time after burn of 12 months (10-14 months), who underwent subcicatricial fat grafting associated with rigotomy were selected. Skin biopsies were performed in before and 14 weeks after operation. The following issues were assessed: 1) esthetic-functional improvement of the scar, by using the Vancouver scar scale; 2) quantitative and qualitative analyses of cicatricial collagen; 3) immunohistochemical analysis of scar vascularization with anti-vascular endothelial growth factor (anti-VEGF) antibody.
RESULTS: When comparing the pre- and post-fat grafting period, a significant esthetic-functional improvement and, microscopically, a redefinition of the boundaries of the papillary and reticular dermis were observed, as well as quantitative reduction and reorganization of collagen, in addition to the decrease of the vascularization of the tissue through immunohistochemical analysis.
CONCLUSION: The basic principle of the whole physiological healing process is to reestablish local homeostasis, that is, excessively intensified steps that lead to severe clinical changes. When subcicatricial fat grafting associated with rigotomy was performed, qualitative and quantitative improvements of the tissue were verified in this study. Thus, it becomes evident that this procedure can complement the treatment of cicatricial pathologies.
Keywords: Skin grafting; Scar; Burns; Healing; Reconstructive surgical procedures.
RESUMO
INTRODUÇÃO: O aumento da sobrevida na fase aguda do paciente grande queimado faz também aumentar a prevalência das sequelas cicatriciais estético-funcionais. Ao pensarmos na relevância clínica da lipoenxertia subcicatricial, necessita-se da compreensão e avaliação microscópica das reais alterações após o procedimento.
MÉTODOS: Foram selecionados oito pacientes, vítimas de queimadura por álcool, com tempo médio após queimadura de 12 meses (10-14 meses), sendo submetidos à lipoenxertia subcicatricial associada à Rigottomy. Foram realizadas biópsias cutâneas no pré e pós-operatório tardio com 14 semanas. Avaliados os quesitos: 1 - Melhora estético funcional da cicatriz pela escala de Vancouver; 2 - Análise quantitativa e qualitativa do colágeno cicatricial; 3 -Análise imunohistoquímica da vascularização cicatricial com anti-fator de crescimento derivado do endotélio vascular (antiVEGE).
RESULTADOS: Ao compararmos o período pré e pós-lipoenxertia, pôde-se avaliar que houve melhora estético-funcional significativa e, microscopicamente, redefinição entre os limites da derme papilar e reticular; redução quantitativa e reorganização do colágeno, além do decréscimo da vascularização tecidual pela análise imunohistoquímica.
CONCLUSÃO: O princípio básico de todo processo cicatricial fisiológico é reestabelecer a homeostasia local, ou seja, as etapas exageradamente intensificadas levam a alterações clínicas catastróficas. Ao realizar a lipoenxertia subcicatricial associada à Rigotomia, foi verificada, neste estudo, a melhora qualitativa e quantitativa do tecido. Sendo assim, torna-se evidente o futuro promissor deste procedimento para a complementação terapêutica das patologias cicatriciais.
Palavras-chave: Enxerto de pele; Cicatriz; Queimaduras; Cicatrização; Procedimentos cirúrgicos reconstrutivos.
The increased survival of patients with extensive burns in the acute phase has led to the increase in the prevalence of scarring sequelae, which increasingly encourages the development of new techniques and procedures for the treatment of these burns.
The large esthetic-functional damage of such sequelae is the result of the scarring disarray induced by thermal trauma1, in which the disorderly stimulus of the scarring stages produces catastrophic results such as hypertrophic and keloid scars, which in most cases, limit articular functioning.
Several studies have quantified the impact of scars on the lives of individuals with burn sequelae by using assessment scales, but the evidence for most of the recommendations for scar therapy is limited1.
This is because the healing process is one of the most complex processes of human metabolism, involving a wide variety of cells and cytokines, in addition to being influenced by external factors.
Carrel, in 1910 (apud Guyton1), classified this process in three phases (inflammation, proliferation, and remodeling), helping us to understand in a simplistic way the healing routine, and this same order occurs throughout the healing process regardless of the tissue type or type of damage2. However, within the complex factors that may affect the final result, the amount of injured tissue and the severity of the injury are known to be the main elements of the disorderly scarring3 present in all burns.
Following the inflammatory response in the healing process, the injured tissue initiates angiogenesis, fibroplasia, and epithelialization, also as a defense against the external environment. This phase is characterized by the formation of granulation tissue composed of a newly formed capillary bed, fibroblasts, macrophages, and an arrangement of collagen, fibronectin, and hyaluronic acid.
Several studies that used growth factors have worked on the idea of modifying this phase of proliferation, especially fibroplasia, which is responsible for most pathological scars after burns4. However, little is known about the factors that decrease the proliferative scar response, that is, what clinically might be helpful in reducing the incidence of scar contracture after burns5.
Still considering the process of formation of so-called pathological scars, the continuity of the inflammatory process, such as in cases of burns, is well known to maintain the stimulation of fibroblast activation, thereby creating an environment sutured in collagen, whose structural arrangement is different from that presented in normal healing6, which results in large scar contractures.
Within this concept, two phenomena seem to be crucial for the elucidation of the actual alterations present in the contractures, namely the organization of collagen fibers and the vascular cicatricial behavior, which reflect the extended inflammatory response4.
Thus, considering ways to supply the scar tissue with some factor that induces a remission or manages local tissue reorganization, Rigotti7 proposed from clinical observations that subcicatricial fat grafting improves the macroscopic and functional quality of scars, generating structural changes during the 14 initial weeks of the scar remodeling process.
Studies that report procedures related to fat grafting are constantly being published8,9. In this case, the technique proposed by these authors7 draws attention to the method of obtaining the fat to be grafted, where non-physical aggression of adipocytes is the rule. Liposuction is performed with 2-mm-thick 12-hole cannulas, with a mean suction pressure of 250 mmHg, followed by centrifugation at low speed (15g) for 3 minutes. For grafting, the rigotomy technique is used, in which several micropunctures are made with an 18-G needle are held on the scar area to create a three-dimensional lipofilling environment4 (Figure 1).
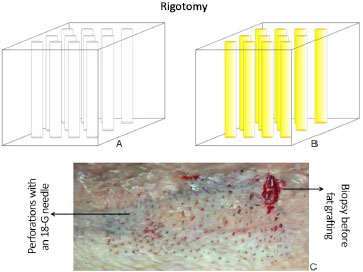
Figure 1. A: Tissue after perforations with an 18-G needle; B: After subcicatricial fat grafting; C: Aspect in vivo of rigotomy.
The theory that was proposed to improve the structural quality of the scar is based on the presence of products derived from stem cells4, but the concept and real contribution of stem cells are still superficially investigated issues.
OBJECTIVES
This study aimed to assess the functional, microscopic, and immunohistochemical changes that occur after subcicatricial fat grafting in burn sequelae by comparing the preoperative and late postoperative periods (14 weeks) in terms of the following:
1. Evaluation of the esthetic and functional aspects of the scar by using the Vancouver scar scale10;
2. Quantitative and qualitative microscopic analyses of scar collagen stained with hematoxylin-eosin and picrosirius; and
3. Immunohistochemical analysis of scar vascularization with anti-vascular endothelial growth factor (anti-VEGF) antibody.
METHODS
After approval by the ethics research committee (CAAE 33848214.9.0000.5430) and obtaining informed consent, we randomly selected eight patients (four men and four women), with a mean age of 35 years (range, 28-61 years), from the Scarring Sequelae Outpatient Clinic of the Department of Plastic Surgery, Faculty of Medicine of Catanduva, SP, Brazil. All the patients had alcohol burns, with an average post-burn time of 12 months (range, 10-14 months), with axillary and/or cervical scar contracture and joint dysfunction by skin sequelae. The authors report no conflicts of interest.
All the patients underwent skin biopsy of the lesion, followed by subcicatricial fat grafting associated with rigotomy, as proposed by Rigotti7 and described in the following section. Then, 14 weeks after fat grafting, they underwent another biopsy 2.5 cm from the previous one but still on the scar (Figure 2).
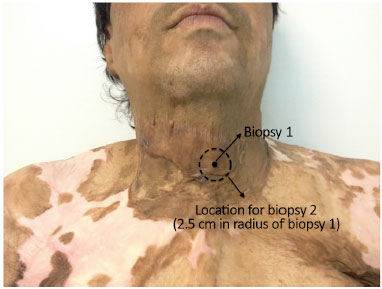
Figure 2. Standardization of cutaneous biopsies before and after fat grafting.
Surgical technique (rigotomy)
Under general anesthesia, tissue was obtained (super-wet technique) from the inferior abdomen, through 2-mm-thick 12-hole liposuction cannulas, with a mean suction pressure of 250 mmHg. The volume obtained was in accordance with the surface to be grafted. Moreover, as described by Khouri et al.7, the entire volume was subjected to manual centrifugation at low speed (15g) for 3 minutes, without manipulating the tissue after centrifugation as recommended by Coleman11.
The scar area to be fat grafted was submitted to micropunctures with an 18-G needle up to the hypodermic level, with an average distance of 5 mm between each hole. With a 2-mm infiltration tube, retrograde and linear distribution of the graft was performed under the scar tissue. After the procedure, local micropore tapes were used to stabilize the subcutaneous graft.
For comparative analysis, two groups were created as follows: before and after fat grafting, with an interval of 14 weeks. At each time point, all the patients were evaluated for the following criteria:
1. Esthetic and functional assessment of the scar, by using the Vancouver scar scale (Table 1), in which the lower scores represent the best quality scarring.
2. Quantitative and qualitative microscopic analyses of scar collagen, in which all the patients underwent spindle biopsy of the scar up to the hypodermic level before and 14 weeks after fat grafting, with a maximum distance between biopsy sites of 2.5 cm (Figure 2).The material was stained with hematoxylin-eosin and picrosirius, and examined under a microscope, with and without polarized light (Leica®), which allowed the assessment of the organization of the collagen site. With the use of the GraphPad Prism® software, collagen was quantified based on the density and light spectrum, being that under polarized light and staining with picrosirius, normal collagen (organized) displays a yellow-greenish color, while fibrotic tissue with disorganized collagen stains red-orange.
3. Immunohistochemical analysis of the scar vascularization, in which the same tissue used in the previous evaluation was now marked with anti-VEGF antibody and analyzed on polarized light. The analysis of VEGF by using the GraphPad Prism® software allowed the quantification of tissue neovascularization at the two time points. The whole process of vascular induction by VEGF represents some degree of local inflammation; that is, the higher the index of neovascularization, the greater the tissue inflammatory response1.The t test and Wilcoxon test were used for the statistical analysis.
RESULTS
The evaluation of the patients at the aforementioned time points, with an interval of 14 weeks, showed significant improvement in the tissue induced by fat grafting. When using the Vancouver scar scale, the scores were reduced in all the cases. In other words, the scars showed esthetic and functional improvements after fat grafting and rigotomy (Figure 3).
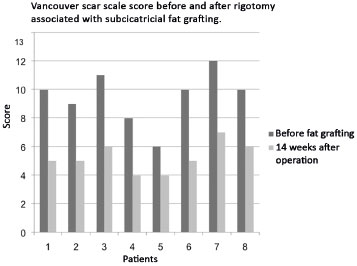
Figure 3. Assessment of patients' Vancouver scar scale scores and evidence of esthetic-functional improvement of scars after fat grafting.
As for microscopic evaluation by using hematoxylin-eosin (Figure 4) and picrosirius staining, a structural improvement of the tissue subjected to fat grafting was observed, both by the redefinition between the papillary and reticular dermis and between the reorganization and reduction (p < 0.05) of the cicatricial collagen measured by interpretation of the light spectra in the GraphPad Prism® software (Figure 5).
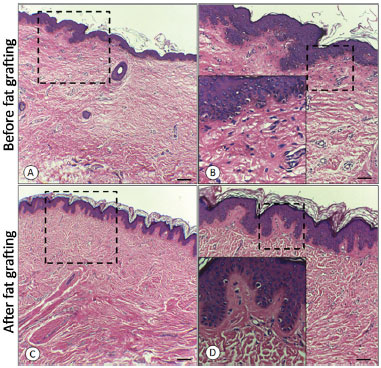
Figure 4. A: Before fat grafting; B: Before fat grafting with amplification of the dermo-epidermal transition; C: After fat grafting; D: After fat grafting with amplification of the dermo-epidermal transition.
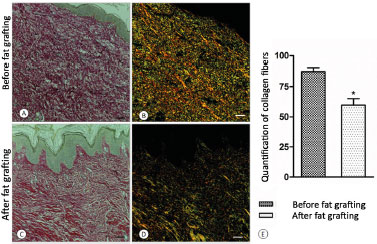
Figure 5. A: Before fat grafting; B: Before fat grafting under polarized light; C: After fat grafting; D: After fat grafting under polarized light; E: Graph of collagen quantification.
In terms of neovascularization, a reduction of the process induced by VEGF was observed between the two periods, with a reduction in tissue vascularization in the fat grafted tissue (p < 0.05; Figure 6) when evaluated by using immunohistochemistry in conjunction with the GraphPad Prism® software.
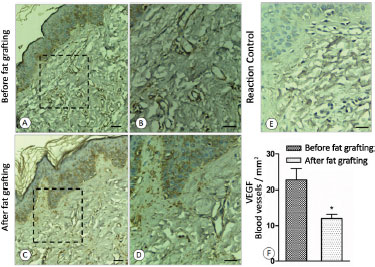
Figure 6. A: Before fat grafting; B: Before fat grafting with amplification; C: After fat grafting; D: After fat grafting with amplification; E: Control of reaction; F: Graph of the concentration of VEGF.
VEGF: vascular endothelial growth factor.
DISCUSSION
The whole healing process is based on deposition and remodeling of collagen fibers. Everything is initiated by an inflammatory response that, in accordance with the area of injury and the severity of the agent, can either intensify these steps or not1.
Some scarring phenomena such as neovascularization depend on the induction factors of the inflammatory response, being more intense when the inflammation persists or is initially very strong2. Thus, scars with an intense inflammatory phase, as in the case of burns, have a rich neovascular network, constantly induced by VEGF (vascular endothelial growth factor). One way of quantifying the inflammatory status of scar tissue would be to assess the degree of neovascularization.
In the case of pathological scars (outcome of burns), the intense neovascularization of the tissue as demonstrated in this study confirms the high inflammatory potential of this tissue. The introduction of fat cells and their residues in the scar interstitium promoted a significant reduction of the inflammatory response and, consequently, in the local pathological vascularization (p < 0.05). The relationship between neovascularization and the inflammatory process is straightforward. It can be assumed that such a vascular decrease after fat grafting is related to a reduction in the local chronic inflammatory process.
As a consequence, one can also observe the structural reorganization of scar tissue by the realignment and reduction of collagen fibers, which translates clinically in functional and macroscopic improvement of the tissue, allowing freedom of articular motion that is previously restricted by the capsular contracture. Furthermore, the reorganization induced by fat grafting allowed a new delineation between the papillary dermis and reticular dermis, which was previously lost.
With regard to the agent inducer of the reorganization and inflammatory reduction present in this tissue, further molecular and genetic studies need to be conducted. However, the simple explanation of the presence of stem cells in adipose tissue needs to be carefully evaluated. In addition, some basic concepts about the subject should be redefined in order to not underestimate other factors of lesser complexity as responsible for the pathological stimulus that triggers the large and dysfunctional scar sequelae.
CONCLUSION
The basic principle of the whole scar healing process is to restore local homeostasis, that is, reintensifying the steps to induce clinical changes.
In the case of scar sequelae of burns, the development of therapies for improvement or resolution of this process provides patients with functional, psychological, and economic gains as they reestablish their occupational activities.
With subcicatricial fat grafting, the qualitative and quantitative improvements of scar tissue were proven in this study, as one can microscopically evaluate the reduction of the inflammatory process and the structural reorganization of the scar by the distinction between the papillary and reticular dermis, in addition to the quantitative reduction of neovascularization and local collagen deposit (p < 0.05).
Therefore, we can conclude that the process of subcicatricial fat grafting associated with the rigotomy technique4 positively changes the microstructure of the scar, presenting macroscopic and microscopic benefits. Thus, it becomes evident that autologous fat grafting can complement the treatment of scar pathologies.
ACKNOWLEDGMENTS
We thank the Department of Plastic Surgery of the Faculty of Medicine of Catanduva, headed by Prof. Dr. Manoel Alves Vidal, which provided the structure for conducting this work, in addition to the Department of Histology/Pathology, represented by Prof. Dr. Ana Paula Girol, who never refrained from the commitment to provide her expertise for the completion of this study.
COLLABORATIONS
AMLAF Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and drafting of the manuscript or critical review of its contents.
BPV Conception and design of the study.
MAV Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and drafting of the manuscript or critical review of its contents.
APG Analysis and/or interpretation of data; statistical analysis; and completion of operations and/or experiments.
HRS Analysis and/or interpretation of data and completion of operations and/or experiments.
MG Completion of operations and/or experiments.
REFERENCES
1. Carrel A. The treatment of wounds. JAMA. 1910; 55:2148. Apud Guyton AC. Guyton and Hall Textbook of Medical Physiology. 10th ed. Philadelphia: Saunders; 2000. p. 385-387.
2. Del Vecchio D, Rohrich RJ. A classification of clinical fat grafting: different problems, different solutions. Plast Reconstr Surg. 2012;130(3):511-22.
3. Durani P, McGrouther DA, Ferguson MW. Current scales for assessing human scarring: a review. J Plast Reconstr Aesthet Surg. 2009;62(6):713-20.
4. Sterodimas A, de Faria J, Nicaretta B, Boriani F. Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: a study. Aesthet Surg J. 2011;31(6):682-93.
5. Lu F, Li J, Gao J, Ogawa R, Ou C, Yang B, et al. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg. 2009;124(5):1437-46.
6. Aiba-Kojima E, Tsuno NH, Inoue K, Matsumoto D, Shigeura T, Sato T, et al. Characterization of wound drainage fluids as a source of soluble factors associated with wound healing: comparison with platelet-rich plasma and potential use in cell culture. Wound Repair Regen. 2007;15(4):511-20.
7. Khouri RK, Rigotti G, Cardoso E, Khouri RK Jr, Biggs TM. Megavolume autologous fat transfer: part II. Practice and techniques. Plast Reconstr Surg. 2014;133(6):1369-77.
8. Bersou Júnior A. Lipoenxertia: técnica expansiva. Rev Bras Cir Plást. 2008;23(2):89-97.
9. Oliveira Neto FV. Enxerto de gordura humana em ratos: Estudo comparativo de diferentes preparos da gordura. Rev Bras Cir Plást. 2011;26(3 Suppl. 1):5.
10. Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16(5):535-8.
11. Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(Suppl):108S-20S.
Faculdade de Medicina de Catanduva, Catanduva, SP, Brazil
Institution: Faculdade de Medicina de Catanduva, Catanduva, SP, Brazil.
Corresponding author:
Auro Marcos Levy de Andrade Filho
Rua Jesus Blanco Nunes, 43 - Santa Marta
São Carlos, SP, Brazil Zip Code 13564-270
E-mail: aurinhu@yahoo.com.br
Article received: May 17, 2015.
Article accepted: August 6, 2016.
Conflicts of interest: none.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter