

Review Article - Year 2016 - Volume 31 -
Intralesional treatment of lymphatic malformations with emphasis on Picibanil (OK-432) sclerotherapy: a systematic review
Tratamento intralesional de malformações linfáticas com ênfase na escleroterapia com picibanil (OK-432): revisão sistemática
ABSTRACT
INTRODUCTION: We performed a retrospective systematic review of studies reporting the use of Picibanil for treatment of lymphatic malformations (LMs).
METHODS: We searched the PubMed database for available studies, including those published between January 1990 and April 14, 2013. The search strategy involved the use of the keywords "OK-432" or "Picibanil" and "lymphatic malformation." Information was compiled regarding the reported mechanism of action, indications, contraindications, efficacy, administration, side effects, complications, and advantages and disadvantages compared to those of other modalities.
RESULTS: Forty-four studies were found, of which 27 fulfilled the inclusion criteria. Picibanil is a lyophilized preparation of a low-virulence strain of Streptococcus pyogenes inactivated with penicillin G. Its mechanism of action is unclear, but it has been speculated that it causes a controlled inflammatory response with adhesion of cyst walls. Picibanil is almost unanimously indicated for the treatment of macrocystic LMs, which show a greater effectiveness response compared to that shown by microcystic or mixed LMs. Picibanil is usually administered by puncturing, either with direct visualization or guided by ultrasound, with the patient under general anesthesia. The most widely used preparation comprises 0.1 mg of Picibanil in 10 mL of saline. Side effects are mostly mild, with pain, swelling, and fever being the most frequently reported.
CONCLUSION: The studies had low scientific evidence. A systematic review found that Picibanil is useful against any LM, with better results in macrocystic lesions. Efficacy was comparable to that of other therapies. No specific contraindication was presented. Although the mechanism of action has not been established, the inclusion of Picibanil as a treatment option is warranted.
Keywords: Lymphatic abnormalities; Therapeutics; Sclerotherapy; Picibanil; Streptococcus pyogenes.
RESUMO
INTRODUÇÃO: Conduziu-se revisão sistemática retrospectiva da literatura incluindo estudos relatando o uso de picibanil para tratar malformações linfáticas (ML).
MÉTODOS: A pesquisa foi realizada com estudos publicados no PubMed de janeiro de 1990 a 14 de abril de 2013. Na estratégia de busca, usou-se os descritores "OK-432" ou "Picibanil" e "lymphatic malformation". Os seguintes elementos foram comparados aos de outras modalidades relatadas e, então, compilados: mecanismo de ação, indicações, contraindicações, eficácia, administração, efeitos colaterais, complicações, vantagens e desvantagens.
RESULTADOS: Foram encontrados 44 estudos, 27 dos quais atenderam aos critérios de inclusão. O picibanil é uma preparação liofilizada de uma cepa de baixa virulência de Streptococcus pyogenes inativada pela penicilina G. Seu mecanismo de ação ainda não definido claramente, mas especula-se que provoque uma resposta inflamatória controlada com adesão das paredes dos cistos. O picibanil é indicado quase que unanimemente para o tratamento da ML macrocística, cuja resposta é mais efetiva do que em lesões microcísticas ou mistas. Em geral, o picibanil é administrado por meio de punção com visualização direta ou guiada por ultrassonografia, com o paciente sob anestesia geral. A preparação comumente utilizada consiste em 0,1 mg de picibanil em 10 ml de soro fisiológico. Os efeitos colaterais são, em geral, leves; sendo dor, inchaço e febre os mais frequentemente relatados.
CONCLUSÃO: Os estudos apresentam pouca evidência científica. A revisão sistemática identificou que o picibanil é útil no tratamento da ML de qualquer tipo, mas tem resultados melhores em lesões macrocísticas. A eficácia foi comparável à de outras terapias. Não foi apresentada nenhuma contraindicação específica. Embora o mecanismo de ação ainda não tenha sido determinado, o picibanil trata-se de opção de tratamento.
Palavras-chave: Anormalidades linfáticas; Terapêutica; Escleroterapia; Picibanil; Streptococcus pyogenes.
Lymphatic malformation (LM), previously known as lymphangioma or cystic hygroma, is a relatively uncommon congenital vascular anomaly1,2. The literature describes this condition as a benign hamartomatous lesion involving the skin and subcutaneous tissue, or as a benign cystic mass arising from abnormal development of lymphatic vessels1-5.
More than half of LMs (50-75%) are clinically present at birth, but symptoms often become more evident during childhood. According to Zhou et al.6, 80-90% of cases not noticeable at birth are diagnosed around the age of 2 years.
LMs are commonly found in the cervicofacial region (75% of cases). The tongue, lips, oral mucosa, and neck are the mainly affected sites3,4,6; other frequently affected sites are the axilla and mediastinum2,5.
LMs are histologically characterized by cysts of varying size, constituted by endothelial cells and filled with lymphatic fluid2. The International Society for the Study of Vascular Anomalies (ISSVA) classification divides LMs into macrocystic, microcystic and mixed lesions. Clinically, LMs present as translucent lesions but may have a reddish or yellowish appearance when mucosal compromise occurs. In addition, lymphatic cysts may contain blood or purulent secretion when accompanied by bleeding or infection6.
In addition to physical disfigurement, impairment of lymphatic flow in LMs can cause severe dysfunction2,4,5. Their progressive growth may cause compression of adjacent vital structures such as the trachea, major vessels, and nerves1,6. When present in thoracic or abdominal spaces, LMs can trigger effusions and ascites, eventually leading to respiratory failure1.
Treatment of LMs includes a variety of procedures based on interventional radiology or surgery. There is no consensus regarding the most effective approach for all clinical presentations. However, intralesional agents seem to be at least less invasive than surgical options.
Several sclerosing agents have been used in the intralesional approach, including hypertonic glucose solution, ethanol, quinine, doxycycline, sodium morrhuate, corticosteroids, bleomycin, and Picibanil. The most commonly used agents nowadays are doxycycline, bleomycin, ethanol, and Picibanil.
Picibanil, also known as OK-432 (Chugai Pharmaceutical Co. Ltd., Japan), is a lyophilized preparation of a low-virulence strain (SU) of Streptococcus pyogenes (also known as S. hemolyticus) inactivated by heating with penicillin G. This drug was originally used as a non-specific immunostimulant in the treatment of malignancies, and especially in digestive and pleural tumors associated with ascites and hydrothorax2,6,7.
Picibanil is not commercially available worldwide and is not currently approved by the US Food and Drug Administration (FDA). It has been studied in Europe, Japan, and South America, and has been recommended by some groups as a primary treatment for LMs owing to its ease of application, absence of scarring, and reduced aggression to adjacent structures2,6,7. However, the exact mechanism of action of this drug has not been fully elucidated2.
The available trials are mostly case series using individual protocols. Consequently, these studies present low levels of scientific evidence, inhibiting a safe recommendation for this treatment method. Systematic review represents an important and useful methodology to provide more consistent data about the efficacy and safety of Picibanil use for the management of LMs.
The main purpose of this study was to perform a systematic review of studies reporting the use of Picibanil for treatment of LMs, including its mechanism of action, indications, contraindications, efficacy, administration, side effects, complications, advantages and disadvantages.
METHODS
The present study was approved by the Ethical Research Committee of the University of Sao Paulo Medical School (approval number 045/14).
A systematic review of studies available in PubMed database was performed, including those published between January 1990 and June 2014. The search strategy used the keywords: "OK-432" or "Picibanil" and "lymphatic malformation."
The inclusion criteria were:
• Articles included case reports, case series, comparative studies, original articles and reviews;For each study and when available, information on the mechanism of action, indications, contraindications, efficacy, administration, side effects, complications, and advantages and disadvantages compared to other treatment options, was collected and analyzed.
• Articles were available in their entirety;
• Articles were published in English;
• Articles dealt with intralesional treatment of soft tissue LM.
RESULTS
Forty-four studies were found according to the search strategy and 27 fulfilled the inclusion criteria. The excluded studies did not have full text available (7 studies), were not written in English (1 study), or were outside the scope of this study (9 studies).
1. Definition: what is Picibanil?
Picibanil, produced exclusively by Chugai Pharmaceuticals (Tokyo, Japan)8, is a preparation from a low virulence strain of Streptococcus pyogenes group A of human origin, pre-treated with benzylpenicillin G and heated. This lyophilized preparation loses its ability to produce streptolysin and consequently its toxic activities, but retains immunopharmacological properties responsible for LM retraction2,3,6,7. Different definitions and classifications of Picibanil have been reported and are described in Table 1.
Picibanil is also known in the literature as OK-432. It is noteworthy that although most authors preferentially use the "OK-432" nomenclature (19/21 articles), both terminologies ("OK-432" and "Picibanil") are presented in all the articles we reviewed (Table 1).
2. Mechanism of action
Treatment with Picibanil was introduced by Peter et al. in 1987, and according to these authors it has been shown to be a safe therapeutic modality, since adverse events are less frequent than with surgery2,9.
The exact mechanism of action of Picibanil is not yet fully understood, but it is assumed to cause a controlled inflammatory response with cytotoxic effects leading to fibrosis, collapsing of the LM walls, cyst obstruction, and resolution3,8. The effect on the wall cysts is considered a sclerosing effect. Picibanil has been used in LM treatment based on its sclerosing action7, and is recommended as an elective treatment by many authors2.
According to published studies, Picibanil administration produces an increase in natural killer cells, lymphokine-activated killer (LAK) cells, and even CD3 lymphocytes and macrophages. Furthermore, it is believed that this drug stimulates release of interferons (alpha, beta and gamma), TNF (tumor necrosis factor) and interleukins (IL1, IL2, and IL6)1-3. The combination of these factors increases the endothelial permeability of LMs and favors lymphatic drainage. Consequently, the cystic spaces become empty, leading to collapse and sclerosis, resulting in a decrease in lesion size7,10. Other studies claim that Picibanil's action remains confined within the lesion and consists of a non-specific immunostimulation, which includes activation of endothelial cells, resulting in obliteration of lymphatic channels with minimal local fibrosis6. Table 2 summarizes the information regarding the mechanism of action of this drug.
3. Indications
Picibanil was unanimously indicated in macrocystic lesions. Studies that exclusively use this drug in microcystic injuries are less common in the literature. Among the 23 clinical or meta-analytical studies, 19 contained information on indication for Picibanil use, and it was indicated exclusively for the treatment of macrocystic lesions in 248 patients, for isolated microcystic LM in 92 patients, and for mixed lesions in 27 cases (Table 3). Patients usually present complete resolution or favorable responses to treatment in macrocystic LM, irrespective of lesion size. While some authors report poor outcomes in microcystic LMs10-16, others claim that repeated Picibanil injections can achieve good results1,14.
4. Contraindications
The use of Picibanil is formally contraindicated in patients with a history of allergy to beta-lactams due to risk of anaphylactic reactions7,10. Surprisingly, this contraindication was only mentioned in eight of 27 studies (29.2%).
Some authors consider that a lack of effective response after several injections is considered a contraindication for future attempts. Hong et al. stated that if after three attempts no response is observed, a contraindication for further injections is defined4.
5. Effectiveness
Ogita .11 first confirmed the successful treatment of LMs with Picibanil in 1996. Several studies have demonstrated the great efficacy of this drug, particularly for the treatment of macrocystic LM12. Macrocystic LM patients generally exhibit good or complete response to treatment, with resolution regardless of the size of the lesion13. On the other hand, patients with microcystic or mixed LM (macro- and microcystic) do not show satisfactory results13. Lymphatic-venous malformations also showed poor response when compared with pure LM13.
Claesson and Kuylenstierna14 described their experience in the treatment of 32 LM patients (28 children, three teenagers and one adult). The results were excellent in all 28 macrocystic LM patients, except for one, who had previously been treated with ethanol. Moreover, none of the four patients with microcystic LMs required additional therapy, and, for two of them, results were considered excellent. An additional study by Wheeler et al.16 conducted a chart review of seven children undergoing Picibanil therapy. Four children had lesions involving the axilla and/or chest wall, two had involvement of extra-mylohyoid neck tissues and only one child had an LM involving the tongue, floor of the mouth and an extra-mylohyoid component. The authors concluded that macrocystic lesions showed excellent response to Picibanil therapy but that the effectiveness for microcystic lesions was disappointing.
Mello-Filho et al.15, performed a retrospective study of six children diagnosed with head and neck LM treated with Picibanil. The most frequently encountered type was macrocystic LM. All patients showed regression of the mass and in three patients there was complete resolution.
In 2006, Peters et al.13 reported a series of 12 patients, of whom six had macrocystic malformations and six microcystic or mixed venous-lymphatic malformations. Ten abnormalities were located in the head and neck and the remaining two in the extremities. All patients with macrocystic abnormalities showed complete resolution or good response to treatment with Picibanil, without additional treatment. In contrast, those with microcystic malformations or mixed lymphatic-venous lesions responded poorly. Interestingly, the size and location of the lesion did not correlate with treatment response.
Poldervaart et al.3 performed a literature search of studies in English with five or more LM patients who had never been treated before. The results showed that 27% of microcystic LM patients showed an excellent result (i.e. more than 90% regression); 33% a good result (reduction by more than 50%) and 40% a poor outcome (less than 50% reduction). In contrast, studies dealing with macrocystic LM showed 88% excellent results. Similarly, Churchill et al.5 conducted a literature review of LM treatment with Picibanil sclerotherapy in pediatric patients (n = 318 cases). Sixty-six percent of patients with macrocystic lesions had excellent results with sclerotherapy, while only 23% of patients with microcystic lesions showed the same results.
Although LM is congenital and usually located in the cervicofacial region, satisfactory responses could be observed in any location, including large retroperitoneal lesions, without limitation with respect to age10 (Table 4).
Ono et al.1 reported successful Picibanil treatment of two patients with microcystic LM and pleural effusion or ascites. In the first case, a newborn had a large microcystic LM occupying the abdominal cavity, surrounding the portal vein and extending to the retroperitoneum. At 5 months of age, the child developed a large pleural chylous effusion and ascites that was drained, and 0.1 mg of Picibanil (10 mL) was injected through the drainage tube into the abdominal cavity and into the pleural cavity through thoracoscopy.
The ascites and pleural effusion gradually decreased over the next two months. After six months, the child showed no pleural effusion or ascites, despite the persistence of abdominal microcystic LM. The second case was a 26-year-old patient who presented chylous ascites and diffuse microcystic LM in groin and thigh. Through laparoscopy, 0.1 mg Picibanil (10 mL) was injected directly into the retroperitoneal LM. The chylous ascites resolved completely after 2 months and the patient remained stable without ascites for five years1.
Although the above studies support the efficacy of OK-432, evidence for its effectiveness has not been gathered from randomized controlled trials. There is no study that objectively evaluates success, for instance by an observer who is independent from the treating physician, to increase clinical evidence.
6. Administration
Skin testing for beta-lactam allergy is recommended before administration6.
In children, the procedure is usually performed under general anesthesia3,12 and the most widely used preparation is 0.1 mg of Picibanil mixed with 10 mL of saline3,10,11. Airway patency must be maintained by endotracheal intubation during sclerotherapy of extensive cervicofacial LMs involving the tongue, floor of mouth, soft palate, or parapharyngeal region, because edema may develop and obstruct the airway6.
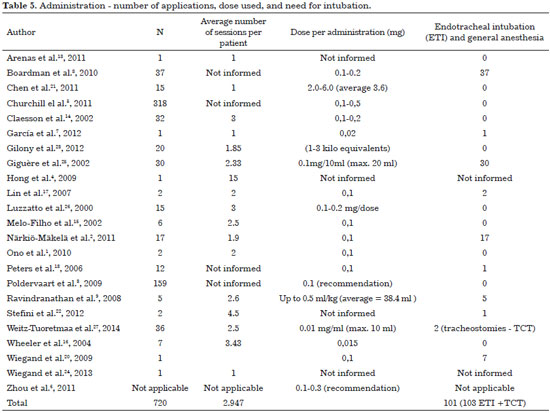
The most widely used access is ultrasound-guided direct puncture. After the contents of the lesion are aspired, the needle (gauge 7)6 must be left in place for injection of the same volume of Picibanil solution3,12. This injection is usually performed at different points and in different directions until cystic expansion is achieved. Most studies advocate not exceeding a total amount of 20 mL6,10. Table 5 describes the average number of applications, doses and indication for tracheal intubation.
According to García et al.7, a new treatment session, when indicated, is performed between 3 and 6 weeks after the first procedure. The dose is increased to 0.3 mg (30 mL). Afterwards, if additional treatment is necessary, the appropriate interval is 1-1.5 months. Other authors who suggest a therapeutic dose of 0.02 mg of Picibanil suggest a second dose of the same amount, after 10-15 days.
Picibanil use in microcystic LM is controversial since there are reports of unsuccessful results in the treatment of this kind of lesion12. Other authors, however, claim that repeated intralesional Picibanil injections achieve good results6. In these cases, the injection is not considered fully intracystic and Picibanil effects can occur within the microcysts or in the lesion interstitium.
7. Side effects and complications
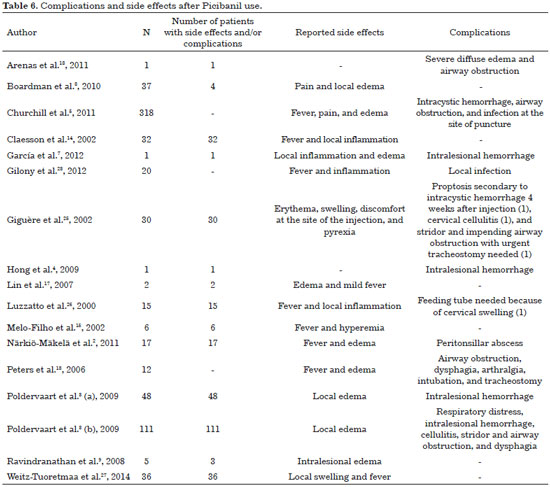
Reported side effects can be categorized as local or systemic (Table 6). Pain, swelling and erythema with 3-7 days duration are frequently reported2,6,12. Almost all patients experience a slight degree of hyperthermia (38-39 °C) up to 6 hours after injection, which improves after 2-4 days. In the literature review conducted by Poldervaart et al.3 in 2009, almost all patients (48) had fever (38-39 °C) associated with local inflammation and lethargy after treatment. These adverse events disappeared in about one week.
Other complications have been also described, including cervical cellulitis and proptosis requiring urgent decompression, cervical abscess requiring surgical drainage and injury to adjacent nerves, and skin necrosis over the LM9. When cystic cavities are very small, the sclerosing agent can be injected, imperceptibly, into surrounding tissue. Extravasation may also be inevitable and may ultimately produce damage to nerves and adjacent healthy tissues17.
Segado Arenas et al.18, in 2011, reported a case of a tongue microcystic LM in a five-year-old boy, still in an early stage of Picibanil infiltration, who presented with severe diffuse edema, progressive obstruction of the upper airway, and respiratory distress, requiring emergency tracheal intubation. The authors concluded that although Picibanil injections are considered safe and effective as a treatment of choice for LM, local edema with potentially fatal airway compromise should be considered.
Although advantageous for its ease of application and absence of scarring, local inflammatory reactions make Picibanil controversial for specific sites7.
DISCUSSION
Lymphatic malformations are defined as low-flow vascular malformations. Microcystic, macrocystic and mixed subtypes have different behaviors in response to various treatment options. Outdated descriptive terms as cystic hygroma, lymphangioma or cavernous malformations must be avoided.
Microcystic LM has a large amount of fibrous connective tissue between small cysts. It tends to be more diffuse, poorly defined, with digitiform protrusions into adjacent tissues. It is difficult to treat by sclerotherapy or to remove completely by surgery. Macrocystic LM tends to be better defined and often responds better to sclerotherapy, regardless of the affected area.
LMs are most commonly located in the cervicofacial region. De Serres et al.19 proposed a classification for cervicofacial LM with therapeutic implications, correlating location and severity.
De Serres Classification:
• Stage 1: Unilateral Infrahyoid;The tongue, lips, oral mucosa and oral floor are mainly affected20. Special attention should be given to extensive lesions involving the floor of the mouth, oropharynx and neck, which may compromise airway patency either by their anatomic location or as a consequence of management. Delayed21 tracheal intubation is advocated by some authors after sclerotherapy for extensive microcystic LM to overcome the expected airway obstruction caused by inflammatory edema3,6,12.
• Stage 2: Unilateral Suprahyoid;
• Stage 3: Unilateral Suprahyoid and Infrahyoid;
• Stage 4: Bilateral Suprahyoid.
The evolution of LM can lead to macroglossia, tongue protrusion, bone deformities, and orthodontic abnormalities such as mandibular prognathism, malocclusion and esthetic deformities. Functional impairment of breathing, chewing, swallowing and speaking as well as psychological problems can occur as a consequence. LMs are unstable lesions, and can grow rapidly after infection, trauma, radiation therapy, bleeding or changes in hormonal levels.
Spontaneous remission has been described in the literature, but can be followed by recurrence. Hence, the most judicious approach is to indicate a treatment modality that has a rapid and effective response.
Historically, treatment for LM was limited to surgical excision. Although this treatment is reasonably effective, poor lesion demarcation, close association with adjacent vital structures, high recurrence rates and risk of complications motivated the development of less invasive approaches22-24.
Thus, the development of therapeutic alternatives, in recent decades, is important. Although several sclerosing agents have been described, Picibanil has emerged in the literature as an effective option with regard to LM sclerotherapy. Picibanil and OK-432 have been used as synonyms in almost all studies, but the term OK-432 predominates.
The exact mechanism of action remains unclear since the hypotheses can be pooled into two partially contradictory theories that need to be better clarified: increased endothelium permeability causing drainage or absorption of cystic contents7,10; and endothelium response by obliteration or sclerosis of malformed lymphatic channels with minimal fibrosis3,6,7,8.
Patients who do not show complete resolution after sclerotherapy with Picibanil are candidates for surgery. Picibanil pretreatment does not compromise surgical dissection. Since there is no method that can provide optimal lymphatic malformation treatment with full resolution in 100% of cases, multimodal therapy, including a combination of sclerotherapy and surgery, should be available as a treatment option.
Reported adverse events after Picibanil sclerotherapy are less frequent, compared with surgery or other sclerosing agents. However, the published studies have a low level of evidence. Prospective controlled trials are necessary to define the real status of long-term effects and efficacy25-27.
Damage to nerves and adjacent tissues may occur when treating microcystic LMs by sclerotherapy. Tissue ischemia and neurotoxicity does not appear to occur with Picibanil. Transient changes in facial nerve function following Picibanil application have been reported after injections in the parotid region. Stretching and compression of nerve branches are considered the causative mechanisms17. Overdose effects related to absorption of Picibanil, such as cardiotoxicity, are rare but have been described.
It is noteworthy that this review presents important limitations for the generalization of findings, since most studies consist of case reports, retrospective studies and literature reviews, which do not allow for statistical evaluation of all collected data. However, this represents the extent of the literature currently available to support discussion and practice.
Although studies have individually shown little scientific evidence, this systematic review finds that Picibanil can be used in any lymphatic malformation, although better results are seen in macrocystic lesions, with reported efficacy comparable and even superior to other treatment modalities. No specific contraindication has been presented and, while the mechanism of action is not yet perfectly established, the inclusion of this drug as a treatment option is warranted.
COLLABORATIONS
OHGP Analysis and/or interpretation of the data; statistical analysis; final approval of the manuscript; formulation of the hypotheses and study design; performance of the surgeries and/or experiments; manuscript preparation and critical review of the content.
DCG Analysis and/or interpretation of the data; statistical analysis; final approval of the manuscript; formulation of the hypotheses and study design; performance of the surgeries and/or experiments; manuscript preparation and critical review of the content.
RG Final approval of the manuscript; critical review of its contents.
REFERENCES
1. Ono S, Iwai N, Chiba F, Furukawa T, Fumino S. OK-432 therapy for chylous pleural effusion or ascites associated with lymphatic malformations. J Pediatr Surg. 2010;45(9):e7-10.
2. Närkiö-Mäkelä M, Mäkelä T, Saarinen P, Salminen P, Julkunen I, Pitkäranta A. Treatment of lymphatic malformations of head and neck with OK-432 sclerotherapy induce systemic inflammatory response. Eur Arch Otorhinolaryngol. 2011;268(1):123-9.
3. Poldervaart MT, Breugem CC, Speleman L, Pasmans S. Treatment of lymphatic malformations with OK-432 (Picibanil): review of the literature. J Craniofac Surg. 2009;20(4):1159-62.
4. Hong JP, Lee MY, Kim EK, Seo DH. Giant lymphangioma of the tongue. J Craniofac Surg. 2009;20(1):252-4.
5. Churchill P, Otal D, Pemberton J, Ali A, Flageole H, Walton JM. Sclerotherapy for lymphatic malformations in children: a scoping review. J Pediatr Surg. 2011;46(5):912-22.
6. Zhou Q, Zheng JW, Mai HM, Luo QF, Fan XD, Su LX, et al. Treatment guidelines of lymphatic malformations of the head and neck. Oral Oncol. 2011;47(12):1105-9.
7. García AL, Navarro RB, Cardeñosa AL, Navarro CD. Resultado sin éxito en el tratamiento de un linfangioma orbitario con OK-432. Arch Soc Esp Oftalmol. 2012;87(1):17-9.
8. Boardman SJ, Cochrane LA, Roebuck D, Elliott MJ, Hartley BE. Multimodality treatment of pediatric lymphatic malformations of the head and neck using surgery and sclerotherapy. Arch Otolaryngol Head Neck Surg. 2010;136(3):270-6.
9. Ravindranathan H, Gillis J, Lord DJ. Intensive care experience with sclerotherapy for cervicofacial lymphatic malformations. Pediatr Crit Care Med. 2008;9(3):304-9.
10. Cabrera J, Redondo P. Tratamiento esclerosante de las malformaciones vasculares. An Sist Sanit Navarra. 2004;Suppl 1:117-26.
11. Ogita S, Tsuto T, Nakamura K, Deguchi E, Tokiwa K, Iwai N. OK-432 therapy for lymphangioma in children: why and how does it work? J Pediatr Surg. 1996;31(4):477-80.
12. Breugem CC, Courtemanche DJ. Portable ultrasound-assisted injection of OK-432 in lymphatic malformations by the plastic surgeon. J Plast Reconstr Aesthet Surg. 2008;61(10):1269-70.
13. Peters DA, Courtemanche DJ, Heran MK, Ludemann JP, Prendiville JS. Treatment of cystic lymphatic vascular malformations with OK-432 sclerotherapy. Plast Reconstr Surg. 2006;118(6):1441-6.
14. Claesson G, Kuylenstierna R. OK-432 therapy for lymphatic malformation in 32 patients (28 children). Int J Pediatr Otorhinolaryngol. 2002;65(1):1-6.
15. Mello-Filho FV, Tone LG, Kruschewsky LS. O uso de Picibanil (OK-432) no tratamento do linfangioma de cabeça e pescoço. Rev Bras Otorrinolaringol. 2002;68(4):552-6.
16. Wheeler JS, Morreau P, Mahadevan M, Pease P. OK-432 and lymphatic malformations in children: the Starship Children's Hospital experience. ANZ J Surg. 2004;74(10):855-8.
17. Lin PJ, Guo YC, Lin JY, Chang YT. Facial nerve conduction after sclerotherapy in children with facial lymphatic malformations: report of two cases. Tohoku J Exp Med. 2007;211(4):401-6.
18. Segado Arenas A, Flores González JC, Rubio Quiñones F, Quintero Otero S, Hernández González A, Pantoja Rosso S. Obstruction iatrogène sévère de la voie aérienne par un lymphangiome lingual. Arch Pediatr. 2011;18(9):983-6.
19. de Serres LM, Sie KC, Richardson MA. Lymphatic malformations of the head and neck. A proposal for staging. Arch Otolaryngol Head Neck Surg. 1995;121(5):577-82.
20. Wiegand S, Eivazi B, Zimmermann AP, Neff A, Barth PJ, Sesterhenn AM, et al. Microcystic lymphatic malformations of the tongue: diagnosis, classification, and treatment. Arch Otolaryngol Head Neck Surg. 2009;135(10):976-83.
21. Chen WL, Huang ZQ, Chai Q, Zhang DM, Wang YY, Wang HJ, et al. Percutaneous sclerotherapy of massive macrocystic lymphatic malformations of the face and neck using fibrin glue with OK-432 and bleomycin. Int J Oral Maxillofac Surg. 2011;40(6):572-6.
22. Stefini S, Bazzana T, Smussi C, Piccioni M, Frusca T, Taddei F, et al. EXIT (Ex utero Intrapartum Treatment) in lymphatic malformations of the head and neck: discussion of three cases and proposal of an EXIT-TTP (Team Time Procedure) list. Int J Pediatr Otorhinolaryngol. 2012;76(1):20-7.
23. Gilony D, Schwartz M, Shpitzer T, Feinmesser R, Kornreich L, Raveh E. Treatment of lymphatic malformations: a more conservative approach. J Pediatr Surg. 2012;47(10):1837-42.
24. Wiegand S, Eivazi B, Bloch LM, Zimmermann AP, Sesterhenn AM, Schulze S, et al. Lymphatic malformations of the orbit. Clin Exp Otorhinolaryngol. 2013;6(1):30-5.
25. Giguère CM, Bauman NM, Sato Y, Burke DK, Greinwald JH, Pransky S, et al. Treatment of lymphangiomas with OK-432 (Picibanil) sclerotherapy: a prospective multi-institutional trial. Arch Otolaryngol Head Neck Surg. 2002;128(10):1137-44.
26. Luzzatto C, Midrio P, Tchaprassian Z, Guglielmi M. Sclerosing treatment of lymphangiomas with OK-432. Arch Dis Child. 2000;82(4):316-8.
27. Weitz-Tuoretmaa A, Rautio R, Valkila J, Keski-Säntti H, Keski-Nisula L, Laranne J. Efficacy of OK-432 sclerotherapy in treatment of lymphatic malformations: long-term follow-up results. Eur Arch Otorhinolaryngol. 2014;271(2):385-90.
Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, SP, Brazil
Institution: Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, SP, Brazil.
Corresponding author:
Dov Goldenberg
Rua Arminda 93 cj. 121
São Paulo, SP, Brazil Zip Code 04545-100
E-mail: dov.goldenberg@hc.fm.usp.br
Article received: February 10, 2016.
Article accepted: June 23, 2016.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter