

Reviw Article - Year 2016 - Volume 31 -
Adipose tissue-derived stem cell autologous grafts: a new approach to application in the treatment of burn victims and reconstructive plastic surgery
Enxerto autólogo de células-tronco derivadas do tecido adiposo: uma nova visão de sua aplicação no tratamento de queimados e na cirurgia plástica reparadora
ABSTRACT
In Brazil, 1 million burn accidents occur annually, and subsequent wound infections account for 75% cases of deaths among these patients, in addition to inducing deformities in the affected areas. Therefore, the aim of this study was to discuss the current status of mesenchymal stem cells, with an emphasis on adipose-derived stem cells (ADSCs), in combination with plasma gel, glue fibrin, and membranes (scaffold). The use of gels and membranes supports cell growth, and aims at potential application in reconstructive plastic surgery for the treatment of burn patients or individuals requiring skin grafts. This study explores and discusses the role of mesenchymal stem cells, adipose-derived mesenchymal stem cells, glue fibrin, plasma gel, and the scaffold. This research collected information from the Virtual Health Library (VHL) and PubMed. A considerable number of articles have been published on burn treatment. However, there is little research on burn treatment with ADSCs, glue fibrin, plasma gel, and scaffold. An ADSC autograft combined with a biological dressing is promising in reconstructive plastic surgery for the treatment and recovery of burn patients or individuals with other injuries that require skin grafts. These features can reduce pain and aid in drying of the lesion, thus promoting neovascularization and wound reepithelialization.
Keywords: Stem cells; Reconstructive surgical procedures; Bioprostheses; Skin; Adipose tissue.
RESUMO
No Brasil, 1 milhão de acidentes com queimaduras acontecem por ano e as infecções são responsáveis por 75% dos óbitos nestes pacientes, além de deixar lesões que ocasionam deformidades nas áreas atingidas. Sendo assim, o objetivo deste trabalho é fornecer uma visão atual sobre células-tronco mesenquimais (MSCs), com ênfase nas células-tronco derivadas do tecido adiposo (ADSCs), associadas a gel de plasma, gel de fibrina e membranas (scaffold). O uso de géis e membranas tendem a auxiliar o crescimento celular visando sua possível aplicação na Cirurgia Plástica Reparadora para o tratamento pacientes queimados ou que necessitam de enxerto de pele. O presente trabalho abordou de forma exploratória e narrativa o tema células-tronco mesenquimais, células-tronco mesenquimais derivadas do tecido adiposo, gel de fibrina, gel de plasma e scaffold. O tipo de pesquisa empregada foi conduzido com coleta de informações utilizando-se a Biblioteca Virtual em Saúde (BVS) e PubMed. O número absoluto de artigos publicados relacionados ao tratamento de queimaduras é considerável. Até o momento, a quantidade de pesquisas relacionadas à terapia com células-tronco derivadas do tecido adiposo, gel de fibrina, gel de plasma e scaffold para o tratamento de queimaduras apresenta-se escassa. O autoenxerto de ADSCs associado a biocurativos torna-se uma perspectiva promissora na Cirurgia Plástica Reparadora para o tratamento e recuperação de pacientes que sofreram queimaduras ou outros acidentes que necessitam de enxerto de pele. Estes recursos podem reduzir a dor e prover a dessecação da lesão, promovendo neovascularização e a reepitelização da ferida.
Palavras-chave: Células-tronco; Procedimentos cirúrgicos reconstrutivos; Bioprótese; Pele; Tecido adiposo.
Among burn cases reported in Brazil, estimates indicate that most incidents occur at home and almost half involve children1. Recently, the World Health Organization (WHO)2 stated that burns cause the death of 195,000 individuals every year, and nearly 11 million people sustain severe burns that require medical care.
In addition, subsequent wound infections are responsible for 75% cases of deaths among burn patients, since the loss of skin protection and presence of devitalized tissues provide an excellent environment for the growth and proliferation of microorganisms, favoring the occurrence of bacteremia and septicemia3. According to estimates of the Ministry of Health4, recent data in Brazil show that the average cost for the treatment of burn patients who require hospitalization reaches R$ 1 million per month.
A variety of dressings is commercially available for the treatment of chronic injuries, with the aim at promoting neovascularization, formation of granulation tissue, and wound closure. First-generation dressings use biomaterials, and second-generation dressings use these in combination with stem cell therapy5.
Skin grafting is one of the procedures used to treat people who sustain burns or other injuries involving tissue loss. The Brazilian Skin Bank accepts donations of cadaver skin to be used in subsequent grafting. However, the availability of homologous skin is still very limited, and such grafts have the disadvantage of only covering the wound temporarily6. Therefore, grafts capable of permanently covering the affected area without being rejected by the recipient are needed. In this context, autologous grafts are ideal for treatment of large defects.
Recently, mesenchymal stem cells (MSCs) have proven to be of great value in protocols for tissue repair because of their rapid proliferation in vitro, and can be reproducibly isolated and manipulated7. Seeking better treatments, the use of autologous grafts with adipose-derived stem cells (ADSCs) has become a promising strategy in Tissue Engineering and Reconstructive Plastic Surgery. These cells have the advantage of differentiating into epithelial tissues, including dermis and epidermis8. In addition to plasticity, ADSCs have remarkable ability to significantly increase granulation tissue and epithelialization, resulting in the acceleration of wound closure9.
OBJECTIVE
Owing to challenges in the treatment of patients with large burns or skin loss caused by other accidents, the aim of this literature review was to identify and discuss studies on MSCs, with emphasis on ADSCs. The combined use of ADSCs and plasma gel, glue fibrin, and membranes (scaffold) to support cell growth will be presented, aimed at assessing their potential application in reconstructive plastic surgery, for the treatment and recovery of patients with burns or other injuries requiring skin grafts.
METHODS
This study investigated the topic of mesenchymal stem cells and mesenchymal stem cells derived from adipose tissue. This research was performed using the Virtual Health Library (VHL) (http://www.bireme.br/php/index.php) and PubMed-NCBI (http://www.ncbi.nlm.nih.gov/pubmed) databases.
The following keywords were used to search for articles: Burn wound; Treatment burn wound; Adipose derived stem cells; Burn wound and cell therapy; Adipose stem cell and glue fibrin; Burn wound and adipose stem cell; Adipose stem cell and plasma gel; Adipose stem cell and scaffold and burn; Adipose stem cell and scaffold and glue fibrin; Adipose stem cell and scaffold and plasma gel. Articles published between 2005 and 2015 were selected, based on these keywords.
RESULTS
The fields of skin wounds and cell therapy have grown in recent years. This is confirmed by the number of publications reviewing survey that were retrieved in the PubMed and VHL databases. From 2005 to July 2015, 20,639 publications related to Burn wound were retrieved from both websites; there were 14,950 articles related to Treatment burn wound, 6,666 related to Adipose derived stem cells, and 1,376 related to Burn wound and cell therapy.
Conversely, few works were related to Adipose stem cell and glue fibrin; Burn wound and adipose stem cell; Adipose stem cell and plasma gel; Adipose stem cell and scaffold and burn; Adipose stem cell and scaffold and glue fibrin; Adipose stem cell and scaffold and plasma gel. Figure 1 shows the absolute distribution of these data in numbers.
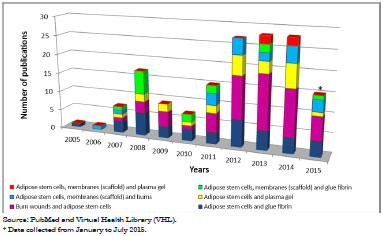
Figure 1. Total annual distribution of limited terms that are related to the treatment of skin wounds and cellular therapy (2005-2015).
DISCUSSION
MSCs and ADSCs
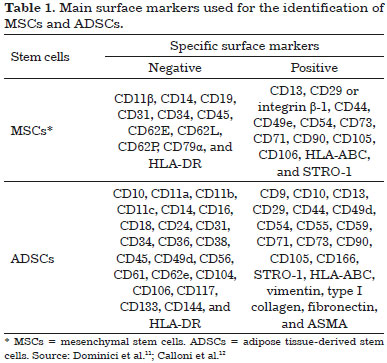
MSCs have been isolated and characterized in different tissues of the body, such as the bone marrow, umbilical cord, brain, epithelium, tooth pulp, and adipose tissue10. MSCs express specific surface markers (Table 1) and are capable of differentiating into multiple lineages of mesodermal cells; however, it was recently observed that MSCs can also differentiate into other cell types that are not mesodermal11,12. MSCs have great advantages because of easy isolation and capacity for proliferation in culture; they are not immunogenic, and can be employed in autologous transplantation13.
Among the MSCs, ADSCs have aroused great interest in reconstructive plastic surgery, as these cells are abundant and can be collected by liposuction, with little risk of morbidity and mortality to the donor9. Figure 2 shows a representative scheme for the breakdown and recovery of the vascular stromal fraction (VSF), with the aim of treating burns and other injuries involving skin loss. ADSCs are located in a niche in the perivascular region formed by blood vessels in association with connective tissue, adipose and stromal cells, various progenitors, and stem cells (Figure 2)10. The VSF is obtained after extensive washing and enzymatic digestion. Besides adult stem cells, the VSF also includes macrophages, lymphocytes, and endothelial vascular cells, among others14.
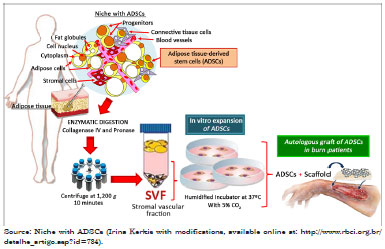
Figure 2. Representative scheme of the experimental manipulation of adipose-derived stem cells (ADSCs) associated with a scaffold (membranes), with the aim of treating burns and other injuries involving skin loss.
Another important feature of ADSCs is their ability to adhere to plastic and proliferate in culture. Thus, cells are isolated through culture and characterized by specific surface markers (Table 1)12. Because of their plasticity when properly induced, these cells can differentiate into multiple cell lineages, including dermis and epidermis8,15. Thus, the pluripotent characteristics of ADSCs can contribute to new treatments for various diseases, particularly in protocols involving cell therapy, which can be performed with quality and reproducibility.
Perspectives for the treatment of burns and other injuries involving skin loss
The main goals in wound treatment are rapid closure, restoration of proper function, and aesthetic satisfaction. With the aim of seeking new therapies for lesion correction, a number of recent studies have shown that ADSCs constitute a source of mesenchymal stem cells; when used in grafting, these release factors that stimulate angiogenesis and accelerate the wound healing process14,15.
While confirming ADSC pluripotency, Chavez-Munoz et al.16 observed that these cells have the ability to transdifferentiate into epidermal keratinocytes and stratified epidermis. In addition, the authors noted that ADSCs might be used in cell therapy, since they offer epidermal coverage in cases of extensive skin loss. It was subsequently observed by immunohistochemistry and Western blotting that ADSCs, when collected from canine models, introduced into the intradermal region located at wound edges in nude mice, and exposed to low-intensity laser therapy, regenerate skin appendages, stimulate neovascularization, and promote the regeneration of skin histological properties17.
It is clear that even stem cells collected from debrided subcutaneous tissue are able to effectively treat in vivo nude mouse wounds, suggesting that these cells could be employed in wound repair and skin regenerative therapies18. Another relevant aspect observed by immunohistochemistry analysis was the statistically significant increase in density of blood vessels and collagen, and secretion of vascular endothelial growth factor (VEGF) and transforming growth factor beta 3 (TGFb3) in studies with autologous transplants of ADSCs in mice. Such factors enhance angiogenesis, wound healing, and survival and overall thickness of the skin graft, suggesting potential value for reconstructive and repair surgeries19.
Similarly, other investigations showed that ADSCs, when used in murine models, contribute to skin regeneration and activation of stem cells of follicular and epithelial tissues, besides aiding in repair of peripheral innervation20,21. The macroscopic and histological findings in murine models of diabetic mice, which heal with great difficulty, revealed that the use of ADSCs in the injured area led to a significant increase in granulation tissue, epithelialization, and accelerated wound closure8.
Wang et al.22, after isolating ADSCs and associated polypeptide membranes of poly-L-glutamic acid (PLGA) and chitosan (CS) from murine models, observed that grafts using these cells proliferate in layers (scaffold), reducing the time of wound closure. In computed microtomography trials (micro-CT), Lee et al.23 confirmed that the incorporation of stem cells isolated from adipose tissue into polypeptide membranes can be a useful and promising tool in tissue engineering. In addition, it should be noted that cellulose membranes and elastin are biocompatible, cytocompatible, and suitable for use with mesenchymal stem cells, with the aim of use immediately after isolation24.
Recently, a randomized controlled study conducted in patients submitted to major surgical procedures showed that the application of ADSCs to surgical wounds improved closure and achieved satisfactory levels of healing9. The loss of soft tissue is a permanent challenge in Reconstructive Surgery, and the application of ADSCs in vivo, in combination with biocompatible materials that serve as cellular support, showed favorable results in injured mice, suggesting their use as a possible new therapy for skin wounds25,26.
Glue fibrin and plasma rich in platelets associated with stem cells
The gel or glue fibrin (Figure 3) consists of two components that are present in plasma, namely fibrinogen and thrombin. In the presence of calcium, these polymerize to form a fibrin network27. Fibrin is a mesh of clotting factors and adhesive proteins that promotes cell adhesion and interacts with the environment, stimulating the formation of collagen and extracellular matrix28.
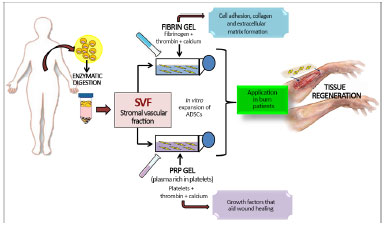
Figure 3. Representative scheme of the experimental manipulation of adipose-derived stem cells (ADSCs) associated with glue fibrin and platelet rich plasma gel (PRP) with the aim of treatment of burns and other injuries involving skin loss.
Commercial preparations approved for clinical use can be used as biological seals. Several experimental studies indicated that glue fibrin plays a role in homeostasis, inflammation, wound healing, and control of angiogenesis29. In addition, many protocols involving tissue engineering have proposed that glue fibrin could be used as a carrier and support for stem cell grafts, since this gel can form a three-dimensional matrix30.
According to Yang et al.31, the association of mesenchymal stem cells with glue fibrin can induce the formation of a scaffold, reproducing the skin structure that allows faster healing. This is a promising strategy, using the graft to replace the skin in the treatment of burn patients. Recently, Taghiabadi et al.32 corroborated this methodology and added that glue fibrin reduced the hypertrophic scar of burn patients, when associated with a keratinocyte culture.
Fibrin is a natural substrate, readily available, abundant in wound healing, and rapidly degraded by the host33. Moreover, the use of glue fibrin can increase the percentage of skin graft acceptance. Evidence also suggests that it improves homeostasis and exerts a protective effect, which results in reduced occurrence of bacterial infection29,32.
It is also worth highlighting plasma gel, which is currently used in tissue engineering protocols (Figure 3), and is obtained by centrifugation of autologous venous blood. This produces a high concentration of platelets in a small volume of plasma, thus leading to plasma rich in platelets (PRP)34. When added to thrombin and calcium, plasma gel is also a blood component that promotes platelet activation and clotting, leading to formation of a platelet gel. This gel is rich in platelet-derived growth factor (PDGF), transforming growth factor alpha1 and beta1 (TGF-alpha1 and beta1), endothelial growth factor (EGF), vascular endothelial growth factor (VEGF), prostaglandin E2 (PGE2), and insulin-like growth factor (IGF)35.
With the current need for substrates and membranes that are not immunogenic and that present proper cell growth factors, in addition to improving vascularization and tissue healing, PRP seems to be a promising vehicle, capable of meeting mesenchymal stem cell requirements36,37. Moreover, it is worth noting that PRP has recombinant growth factors, is pathogen-free, and is from an autologous source, which makes its application more effective and safer for the patient36.
It was observed that the growth factors present in PRP are not mutagenic and act to effectively stimulate healing. PRP might be used alone or in combination with stem cells in tissue regeneration38. Among studies in which stem cells are associated with PRP, it was observed that the receptors expressed by stem cells are activated by the growth factors present in PRP. These induce the upregulation of genes from the extracellular matrix, collagen synthesis, and cell proliferation, thus playing important roles in the healing process39,40.
CONCLUSION
Although there have been advances in procedures for burns and other injuries involving skin loss, the importance of finding new therapies capable of aiding in the recovery of individuals with skin lesions is significant. The aim is to minimize complications, treatment time, and costs, thus ensuring the return of the individual to normal activities as soon as possible.
The combinations of ADSCs with glue fibrin or plasma gel associated with membranes, which aim to provide a scaffold to support cell growth, are promising strategies capable of meeting stem cell requirements. Under these conditions, homeostasis is maintained in tissues that replace cells lost during maturation, aging, or injury, thus providing proper cell growth with the aim of reliably reproducing the lost structure and function of the original skin.
Therefore, autologous grafts using ADSCs, in combination with biological dressings, offer a promising perspective in reconstructive plastic surgery for the treatment and recovery of burn patients or individuals with other types of injuries requiring skin grafts; these grafts can reduce pain and modulate drying in a wound, thus promoting neovascularization and wound reepithelialization.
COLLABORATIONS
CSCG Conception and design of the study; writing of the manuscript or critical review of its content.
LCCG Writing of the manuscript or critical review of its content.
JAPH Writing of the manuscript or critical review of its content.
MRE Writing of the manuscript or critical review of its content.
PMCG Final approval of the manuscript; writing of the manuscript or critical review of its content.
ACKNOWLEDGMENTS
To the Institute of Biotechnology of the University of Caxias do Sul, Coordination for the Improvement of Higher Education Personnel - CAPES and National Council for Scientific and Technological Development - CNPq.
REFERENCES
1. Tavares CS, Hora EC. Caracterização das vítimas de queimaduras em seguimento ambulatorial. Rev Bras Queimaduras. 2011;10(4):119-23.
2. WHO. World Health Organization. Media Centre. Fact sheet n. 365. 2012 [online]. [Acesso 2015 Mai 27]. Disponível em: http://www.who.int/mediacentre/factsheets/fs365/en/index.html
3. Rempel LCT, Tizzot MRPA, Vasco JFM. Incidência de infecções bacterianas em pacientes queimados sob tratamento em hospital universitário de Curitiba. Rev Bras Queimaduras. 2011;10(1):3-9.
4. Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde Departamento de Atenção Especializada. Cartilha para tratamento de emergência das queimaduras [online]. 2012. [Acesso: 2015 Mai 23]. Disponível em: http://sbqueimaduras.org.br/wp/wp-content/uploads/2013/04/Cartilha_MS_2012.pdf
5. Suvarna K, Munira M. Wound Healing Process and Wound Care Dressing: A Detailed Review. J Pharm Res. 2013;2(11):6-12.
6. Schiozer W. Banco de pele no Brasil. Rev Bras Queimaduras. 2012;11(2):53-5.
7. Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318-24.
8. Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20(2):205-16.
9. Martins PDE, Uebel CO, Machado DC, Da Silva JB. Uso de células-tronco adultas de tecido adiposo na cicatrização da pele: estudo controlado, randomizado. Rev Bras Cir Plást. 2009;26(3):394-401.
10. Yarak S, Okamoto OK. Células-tronco derivadas de tecido adiposo humano: desafios atuais e perspectivas clínicas. An Bras Dermatol 2010;85(5):647-56.
11. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7.
12. Calloni R, Cordero EA, Henriques JA, Bonatto D. Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev. 2013;22(9):1455-76.
13. Klinker MW, Wei CH. Mesenchymal stem cells in the treatment of inflammatory and autoimmune diseases in experimental animal models. World J Stem Cells. 2015;7(3):556-67.
14. Banyard DA, Salibian AA, Widgerow AD, Evans GR. Implications for human adipose-derived stem cells in plastic surgery. J Cell Mol Med. 2015;19(1):21-30.
15. Caruana G, Bertozzi N, Boschi E, Pio Grieco M, Grignaffini E, Raposio E. Role of adipose-derived stem cells in chronic cutaneous wound healing. Ann Ital Chir. 2015;86(1):1-4.
16. Chavez-Munoz C, Nguyen KT, Xu W, Hong SJ, Mustoe TA, Galiano RD. Transdifferentiation of adipose-derived stem cells into keratinocyte-like cells: engineering a stratified epidermis. PLoS One. 2013;8(12):e80587.
17. Kim H, Choi K, Kweon OK, Kim WH. Enhanced wound healing effect of canine adipose-derived mesenchymal stem cells with low-level laser therapy in athymic mice. J Dermatol Sci. 2012;68(3):149-56.
18. Natesan S, Zhang G, Baer DG, Walters TJ, Christy RJ, Suggs LJ. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng Part A. 2011;17(7-8):941-53.
19. Zografou A, Tsigris C, Papadopoulos O, Kavantzas N, Patsouris E, Donta I, et al. Improvement of skin-graft survival after autologous transplantation of adipose-derived stem cells in rats. J Plast Reconstr Aesthet Surg. 2011;64(12):1647-56.
20. Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761-71.
21. Wu L, Cai X, Zhang S, Karperien M, Lin Y. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspectives from stem cell biology and molecular medicine. J Cell Physiol. 2013;228(5):938-44.
22. Wang W, Cao B, Cui L, Cai J, Yin J. Adipose tissue engineering with human adipose tissue-derived adult stem cells and a novel porous scaffold. J Biomed Mater Res B Appl Biomater. 2013;101(1):68-75.
23. Lee JW, Kim KJ, Kang KS, Chen S, Rhie JW, Cho DW. Development of a bone reconstruction technique using a solid free-form fabrication (SFF)-based drug releasing scaffold and adipose-derived stem cells. J Biomed Mater Res A. 2013;101(7):1865-75.
24. Alharbi Z, Almakadi S, Opländer C, Vogt M, Rennekampff HO, Pallua N. Intraoperative use of enriched collagen and elastin matrices with freshly isolated adipose-derived stem/stromal cells: a potential clinical approach for soft tissue reconstruction. BMC Surg. 2014;14:10.
25. de Paula AC, Zonari AA, Martins TM, Novikoff S, da Silva AR, Correlo VM, et al. Human serum is a suitable supplement for the osteogenic differentiation of human adipose-derived stem cells seeded on poly-3-hydroxibutyrate-co-3-hydroxyvalerate scaffolds. Tissue Eng Part A. 2013;19(1-2):277-89.
26. Chawla R, Tan A, Ahmed M, Crowley C, Moiemen NS, Cui Z, et al. A polyhedral oligomeric silsesquioxane-based bilayered dermal scaffold seeded with adipose tissue-derived stem cells: in vitro assessment of biomechanical properties. J Surg Res. 2014;188(2):361-72.
27. Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost. 2006;4(5):932-9.
28. Schmoekel HG, Weber FE, Schense JC, Grätz KW, Schawalder P, Hubbell JA. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol Bioeng. 2005;89(3):253-62.
29. Cai M, Zhang J, Guan L, Zhao M. Novel implantable composite biomaterial by fibrin glue and amniotic membrane for ocular surface reconstruction. J Mater Sci Mater Med. 2015;26(3):149.
30. Bonilla Horcajo C, Otero L, Aguayo C, Rodriguez A, Zurita M, Vaquero J. Estudio de la utilidad del gel de fibrina como soporte celular en el trasplante intracerebral de células madre mesenquimales. Trauma. 2009;20(4):243-8.
31. Yang Y, Zhang W, Li Y, Fang G, Zhang K. Scalded skin of rat treated by using fibrin glue combined with allogeneic bone marrow mesenchymal stem cells. Ann Dermatol. 2014;26(3):289-95.
32. Taghiabadi E, Mohammadi P, Aghdami N, Falah N, Orouji Z, Nazari A, et al. Treatment of Hypertrophic Scar in Human with Autologous Transplantation of Cultured Keratinocytes and Fibroblasts along with Fibrin Glue. Cell J. 2015;17(1):49-58.
33. Tanikawa DY, Alonso N, Herson MR, Mathor MB, Caldini EG, Lourenço SV, et al. Ultrastructural evaluation of human keratinocyte growth and differentiation on a fibrin substrate. Acta Cir Bras. 2010;25(6):541-8.
34. Vendramin FS, Franco D, Franco TR. Método de obtenção do gel de plasma rico em plaquetas autólogo. Rev Bras Cir Plást. 2009;24(2):212-8.
35. Garcia RV, Gabrielli MA, Hochuli-Vieira E, Spolidorio LC, Filho JG, Neto FA, et al. Effect of platelet-rich plasma on peri-implant bone repair: a histologic study in dogs. J Oral Implantol. 2010;36(4):281-90.
36. Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010;83(1):1-9.
37. Formigli L, Benvenuti S, Mercatelli R, Quercioli F, Tani A, Mirabella C, et al. Dermal matrix scaffold engineered with adult mesenchymal stem cells and platelet-rich plasma as a potential tool for tissue repair and regeneration. J Tissue Eng Regen Med. 2012;6(2):125-34.
38. Chen WH, Liu HY, Lo WC, Wu SC, Chi CH, Chang HY, et al. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30(29):5523-33.
39. Mehrannia M, Vaezi M, Yousefshahi F, Rouhipour N. Platelet rich plasma for treatment of nonhealing diabetic foot ulcers: a case report. Can J Diabetes. 2014;38(1):5-8.
40. Picard F, Hersant B, Bosc R, Meningaud JP. Should we use platelet-rich plasma as an adjunct therapy to treat "acute wounds," "burns," and "laser therapies": A review and a proposal of a quality criteria checklist for further studies. Wound Repair Regen. 2015;23(2):163-70.
1. Instituto de Biotecnologia da Universidade de Caxias do Sul, Caxias do Sul, RS, Brazil
2. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
Institution: Instituto de Biotecnologia da Universidade de Caxias do Sul, Caxias do Sul, RS, Brazil.
Corresponding author:
Paulo Miguel Celi Garcia
Rua Francisco Getúlio Vargas, 1130
Caxias do Sul, RS, Brazil Zip Code 95070-560
E-mail: cirurplasticapgarcia@terra.com.br
Article received: July 20, 2015.
Article accepted: April 10, 2016.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter