
Case Report - Year 2016 - Volume 31 -
Foreign body reaction to polyamide filling in the face
Reação tipo corpo estranho a preenchimento facial com poliamida
ABSTRACT
Dermal fillers are increasingly used, and the number of complications due to their use is significant. In this work, we report the case of foreign body granulomas due to the facial injection of a polyamide gel, named AqualiftTM, a product not found in scientific literature databases. Clinical, therapeutic and hystopathological aspects are discussed. A warning is made, concerning the use of this substance.
Keywords: Injections intradermal/adverse effects; Biocompatible materials/therapeutic use; Granuloma foreign-body.
RESUMO
O uso de substâncias para preenchimento dérmico é crescente, e o número de complicações devido à sua utilização, significativo. Neste trabalho, relatamos um caso de granulomas de corpo estranho após preenchimento facial com gel de poliamida, chamado AqualiftTM, produto não encontrável nas bases de dados da literatura científica. São discutidos aspectos clínicos, terapêuticos e histopatológicos. Faz-se uma advertência relativa ao uso desta substância.
Palavras-chave: Injeções intradérmicas/efeitos adversos; Materiais biocompatíveis/uso terapêutico; Granuloma de corpo estranho.
The use of dermal fillers has increased enormously in recent years; in the USA alone, more than 1.6 million procedures were performed in 20111. The results of their use can be very rewarding; however, they are by no means exempt of adverse effects, which range from simple, transient bruising to complete blindness2. Long-standing nodular complications can be particularly annoying: chronic, torpid nodules at the injection sites, which can eventually drain spontaneously3.
We present one case of foreign body granuloma complications brought by the dermal and subdermal injection, performed by a dentist, of the product AqualiftTM (polyamide gel). No reports of either complications or even of the use of this substance could be found in the medical databases searched by us.
CASE REPORT
A 63 year old white female patient sought us, complaining of lumps on the nasolabial folds and lips, where AqualiftTM had been injected four months ago (Figure 1A). Two months after the injection, the lumps began to appear, being associated to inflammatory symptoms and signs: constant, significant pain, redness and increased local temperature. Some nodules drained spontaneously. The professional who made the injections tried to treat the problems thorough oral antibiotics and steroids, needle aspiration and steroid injections, performed at several sessions, without any improvement. The patient was highly dissatisfied, and desired the surgical removal of the affected areas, rejecting further conservative measures.
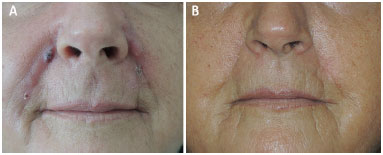
Figure 1. Foreign body granuloma reaction after filling with AqualiftTM; A: Pre-operative photograph; B: Post-operative photograph, 10 months after surgery.
Under local anesthesia with sedation, the excision of the diseased tissues was performed, with magnification to identify the globules of the substance, which were found at the dermis and inside the subcutaneous fat and muscle fibers. Uneventful healing succeeded. The cosmetic result was good (Fig. 1B). The surgical specimens were examined; the report read 'amorphous basophilic material being engulfed by foreign body giant cells, forming granulomas amidst lympho-mono-hystiocytic inflammatory infiltrate, as well as some micro-abscesses in the dermis' (Figure 2A-D).
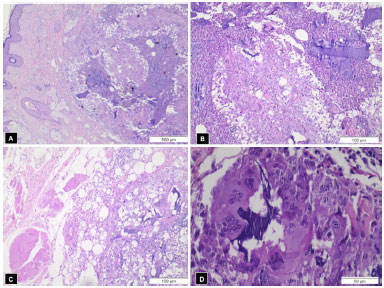
Figure 2. A: Stratified keratinized epithelial tissue (left), with fragments of amorphous basophilic material in the underlying dermis and lympho-mono-histiocytic inflammatory infiltrate (40x); B: Amorphous basophilic material insterspersed with areas of micro-abscesses in dermis (100x); C: Areas of striated muscle and fat tissue (left) with basophilic material and granuloma formation (right) (100 x); D: Giant multinucleated cells phagocyting basophilic material, evidencing foreign body inflammatory reaction (400x).
DISCUSSION
Artificial dermal fillers may be classified, according to their degree of permanency, into permanent (PMMA, polyacrylamide, silicone) and semipermanent (hyaluronic acid, collagen, dextran, polylactic acid and hydroxylapatyte)4,5. Although all of them may generate unwanted effects, these are more frequent and serious when permanent fillers are used6.
True granulomas may appear late, 6-24 months after injection, whereas simple nodules or lumps can be present earlier4, although they have also been observed as late as 24 months after injection of hyaluronic acid7. Nodules are frequently cutaneous, but may present intra-orally as well8. Sometimes, nodules or granulomas are due to substances without known identity, presenting a problem difficult to solve; new spectroscopic methods may be an invaluable aid to identify the filler9.
In the case reported in this work, which was due to foreign body granuloma formation, the filler injected has the commercial name of AqualiftTM; the Brazilian seller's site (www.aqualift.com.br) informed, when this paper was written, that the product would be a hydrophilic gel of polyamide in saline, being made in Ukraine.The product has been licensed by the Brazilian regulatory agency ANVISA in 2013, a license not renewed in 2014; it is not licensed by the American FDA. A thorough search of medical literature led to no results about this filler, although in one reference polyamide has been confounded with polyacrylamide (which is a different substance)10.
Polyamide is more commonly known as nylon, which is the same substance used, since a long time, to fabricate inabsorbable sutures. It is reasonable to consider this product as a permanent filler. This category certainly generates more reactions than temporary agents11.
The nodules, in this case, began to appear only two months after the injection. According to some authors4, true granulomas would arise only after six months. Notwithstanding this, the specimen examination showed that the nodules were, indeed, true foreign body granulomas.
Although many complications of filler use may be due to the technique of injection4, when substances devoid of scientific background are used, it can be hypothesized that side effects, as those reported here, can be linked to the product itself. In the present case, it was not a plastic surgeon which made the injection; however, some sites of Brazilian plastic surgeons offer this filler.
It is not at all unlikely that similar cases may exist, but are not published. As a warning, we recommend avoiding the use of this product, until a significant number of scientific papers are published on its long-term safety, if ever.
COLLABORATIONS
TNM Performance of experiments; performance of experiments; writing and final approval of the manuscript.
LRO Critical revision of the manuscript content.
SMCGA Analysis and interpretation of the data.
ARS Analysis and interpretation of the data; final approval of the manuscript.
REFERENCES
1. Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
2. Kim YJ, Choi KS. Bilateral blindness after filler injection. Plast Reconstr Surg. 2013;131(2):298e-299e.
3. Cassuto D, Sundaram H. A problem-oriented approach to nodular complications from hyaluronic acid and calcium hydroxylapatite fillers: classification and recommendations for treatment. Plast Reconstr Surg. 2013;132(4 Suppl 2):48S-58S.
4. Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006;118(3 Suppl):92S-107S.
5. Vargas AF, Amorim NG, Pitanguy I. Complicações tardias dos preenchimentos permanentes. Rev Bras Cir Plást. 2009;24(1):71-81.
6. Santos CVF, Machado BHB, Pitanguy I. Reações adversas a preenchimento facial definitivo com polimetilmetacrilato. In: 42º Congresso Brasileiro de Cirurgia Plástica; 11-14 Nov 2005. Belo Horizonte, MG, Brasil.
7. Shahrabi Farahani S, Sexton J, Stone JD, Quinn K, Woo SB. Lip nodules caused by hyaluronic acid filler injection: report of three cases. Head Neck Pathol. 2012;6(1):16-20.
8. Shahrabi-Farahani S, Lerman MA, Noonan V, Kabani S, Woo SB. Granulomatous foreign body reaction to dermal cosmetic fillers with intraoral migration. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):105-10.
9. Persichetti P, Palazzolo D, Tenna S, Poccia I, Abbruzzese F, Trombetta M. Dermal filler complications from unknown biomaterials: identification by attenuated total reflectance spectroscopy. Plast Reconstr Surg. 2013;131(4):597e-603e.
10. Evstatiev D. Late complications after injections of hydrogel in the breast. Plast Reconstr Surg. 2004;113(6):1878-9.
11. Rosa SC, Macedo JLS. Reações adversas a substâncias de preenchimento subcutâneo. Rev Soc Bras Cir Plást. 2005;20(4):248-52.
1. Universidade do Vale do Rio Verde - UninCor, Três Corações, MG, Brazil
2. Faculdade de Medicina de Ribeirão Preto, Ribeirão Preto, SP, Brazil
Institution: Sulplast Clínica Cirúrgica e Universidade do Vale do Rio Verde - UninCor - Três Corações, MG, Brazil.
Corresponding author:
Tufi Neder Meyer
Rua Edson Arantes do Nascimento, 201 - Centro
Três Corações, MG, Brasil Zip Code 37410-000
E-mail: tufi@uai.com.br
Article received: June 10, 2014.
Article accepted: January 25, 2015.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter