

Articles - Year 2003 - Volume 18 -
Effects of L-Arginine on in vivo Concentrations of Metabolites in the Blood and in Myocutaneous Flaps with Surgical Scars, in Wistar Rats
Efeitos da L-Arginina sobre as Concentrações in vivo de Metabólitos no Sangue e em Retalho Miocutâneo Contendo Cicatriz Cirúrgica, em Ratos Wistar
ABSTRACT
The epithelialization, contraction and deposition of connective tissue are the mechanisms by which wounds heal. Studies have shown that L-Arginine favors wound healing by increasing the synthesis of collagen. This paper aimed at studying the effects of L-Arginine supplementation on in vivo concentrations of glucose, pyruvate, lactate and ketone bodies in the blood and in myocutaneous flaps undergoing healing. Forty-four white male Wistar rats were distributed in two groups: (G-l/Control) and (G-2/Experiment). The G-1 animals received iso-proteic casein supplementation and the G-2 group received L-Arqinine by gavage daily. Both groups were submitted to pediculated and dorsal myocutaneous flaps. In the post-operative period, each group was subdivided into three subgroups and, after 7, 14 and 30 days) blood and scar tissue samples were collected for analyses of enzymes. The drop in blood concentrations of pyruvate, lactate and ketone bodies on the l4th day suggests higher utilization of these metabolites in peripheral tissues by a possible anabolic action of L-Arginine. The increase of tissue concentrations of leetone bodies in the myocutaneous flap on the 30th; post-operative day in animals treated with L-Arginine food supplementation indicates a probable increase in the utilization of these metabolites by tissues under scarring. The results suggest that L-Arginine supplementation has an effect on serum concentrations of substrates and on shin and muscle scarring.
Keywords: Surgical trauma; metabolism; scarring; L-Arginine; rats
RESUMO
A epitelização, a contração e a deposição da matriz de tecido conjuntivo constituem mecanismos pelos quais as feridas cicatrizam. Estudos demonstraram que a L-arginina favorece a cicatrização, aumentando a síntese de colágeno. Objetivou-se, neste trabalho, estudar os efeitos da suplementação da L-arginina sobre concentrações in vivo de glicose, piruvato, lactato e corpos cetônicos no sangue e retalho miocutâneo em cicatrização.
Quarenta e oito ratos brancos, machos, da linhagem Wistar, foram distribuídos em dois grupos: (G-1/Controle) e (G-2/Experimento). Os animais do G-1 receberam suplementação isoprotéica de caseinato e os do grupo/G-2 de L-arginina, por gavagem, diariamente. Ambos os grupos foram submetidos à confecção de retalho miocutâneo, pediculado e dorsal. No pós-operatório, cada grupo foi subdividido em três subgrupos e, após 7, 14 e 30 dias, foram coletadas amostras de sangue e tecidos cicatriciais para análises enzimáticas. A queda das concentrações sanguíneas, de piruvato, lactato e corpos cetônicos no 14º dia sugere maior utilização desses metabólitos nos tecidos periféricos por possível ação anabólica da oferta de L-arginina. O aumento das concentrações tissulares, no retalho miocutâneo, de corpos cetônicos no 30º dia de pós-operatório nos animais tratados com suplementação alimentar de L-arginina traduz provável aumento de captação desses metabólitos pelos tecidos em cicatrização. Os resultados sugerem que a suplementação de L-arginina tem efeito sobre as concentrações séricas de substratos e sobre a cicatrização cutânea e muscular.
Palavras-chave: Trauma cirúrgico; metabolismo; cicatrização; L-arginina; ratos
The scarring process may be understood as a defense reaction of the body injured by any traumatic agent in order to maintain its integrity. The pracess is used to reestablish a solution of continuity of tissues and consists of: chemotaxy, cellular division, neovascularization, synthesis of extra cellular pratein matrix and scar remodeling(1,2).
Thanks to discoveries in surgical metabology, biochemistry, and nutrition, a new era has come in which we are trying to interfere in molecular biology, influencing on the synthesis of substances responsible for the development of the scarring phenomena. However, at the local level, the principle of "minimum interference" remains, that is, to cause the least possible chemical and surgical trauma to the damaged tissues so that the scarring pracess is completed without meaningfully changing its original cells(3,4).
The scarring process results from a competitive mechanism between collagen synthesis and lysis. Thus, any factor that increases collagenlysis or decreases its synthesis may lead to changes in scarring(5).
Experimental studies have shown that a 1% supplement of Arginine in the diet of rats increased the tensile strength of the damaged tissue and collagen deposition(6).
Pharmacological Arginine doses substantially increased the quantity of hydroxyprolin, present almost exclusively in collagen, in the subcutaneous cellular tissue in humans, showing that Arginine may increase the synthesis of repair collagen(7).
This paper focused on studying, by means of enzymatic methods, the effects of L-Arginine food supplementation on in vivo concentrations of glucose, pyruvate, lactate and ketone bodies in the blood and the myocuraneous flap under the process of scarring.
METHODS
The study was conducted following the International Norms for Biomedical Research in Animals (1990) and in compliance with the Federal Act n°. 6.638, from May 8,1979. Forty-eight albino male Wistar rats (Rattus norvegicus albinus) were used. Their weight ranged from 250 to 300 g. The rats came fram the central animal facility of the Federal University of Ceará and were maintained in the Laboratory of Experimental Surgery of the Department of Surgery of the School of Medicine (UFC) in polypropylene cages, receiving water and suitable food ad libitum up to 12 hours before the beginning of each experiment. The rats were equitably distributed in two groups: Graup 1 (G-1 - Control) and Group 2 (G-2 - Experiment). Each group was subdivided into three subgraups (n=8). After induction of general inhalation anesthesia with diethyl ether, we made a dorsal myocutaneous flap, pediculated in its cephalic extremity and measuring 3x3x3 cm (Figs. 1 and 2). The flap was surured with mono-filament nylon using separate sutures. Povidone-iodine was used for the antisepsis of the surgical field. The animals of the control group (G-1) received food supplementation of calcium caseinate given by gavage (approximately 5% of the total caloric amount (VCT) intake/day for rats with weight in the range selected for this study, that is, 1.0g calcium caseinate dissolved in 3.0 ml of saline(8)) daily. The animals of the experiment group (G-2) also received, by gavage, 5% of the VCT/day - 1.0 g of L-Arginine dissolved in 3.0 ml of saline daily. After 7, 14 and 30 days since the beginning of food supplementation and surgery, a fragmem of the dorsal flap was removed under inhalation anesthetics, and 2.0 ml of arterial blood was collected. The animal was then sacrificed by a more prolonged exposure to the anesthetic.
Fig. 1 - Demarcation of the myocutaneous flap.
Fig. 2 - Myocutaneous flap (detail).
Metabolite blood concentrations in the myocutaneous flap were determined by enzymatic assay methods(9,10,11). In this study, the concentrations of D-glucose, pyruvate, lactate and ketone bodies (hydroxyburyric acid and acetoacetic) were determined. Metabolite concentrations were calculated in µmol/g of fresh tissue or µmol/ml of blood.
The results were expressed as Mean ± S.M.E. (Standard Mean Error). The Mann-Whitney non-parametric test was used for statistical analysis, fixing the significance level in 5% (p < 0.05).
RESULTS
No significam differences in glycemia or glucose concentrations in the myocutaneous flap were observed in any of the times studied (Figs. 3 and 4).
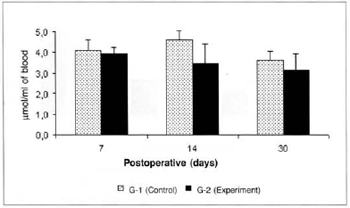
Fig. 3 - Glucose concentration in blood (µmol/ml of blood).
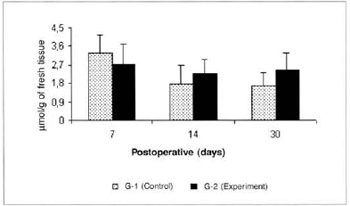
Fig. 4 - Glucose Concentrations in the myocutaneous flap (µmol/g of fresh tissue).
Pyruvate concentrations determined in the blood were significantly lower (0.453 ± 0.12 versus 1.597 ± 0.41), in the comparison between the amounts determined in groups G-2 versus G-l on the 14th postoperative day (Fig. 5). Significant differences in pyruvate concentrations in the myocutaneous flap were not observed in any of the times studied (Fig. 6).
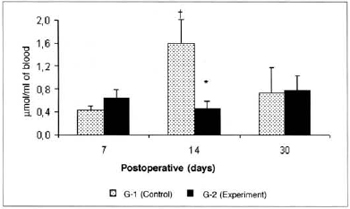
Fig. 5 - Pyruvate Concentrations in blood (µmol/ml of blood). *(p< 0.05) when compared to respective control.†(p< 0.01) when compared to the 7th day in the control group.
Fig. 6 - Pyruvare Concentrations in the myocutaneous flap (µmol/g of fresh tissue).
Lactate concentrations in the blood of the animals of group G-2 were significantly lower (5.103 ± 0.78 versus 7.795 ± 0.51) than the concentrations determined in the respective control (G-l) on the 14th day. A significant increase (7.795 ± 0.51 versus 3.629 ± 0.86) was observed in the lactate concentrations in the blood of the animals of G-2 on the 14th day in comparison with the amounts determined on the 7th post-operative day in the same group (Fig. 7). Significant differences in the lactate concentrations determined in the myocutaneous flap were not observed in any of the times studied (Fig. 8).
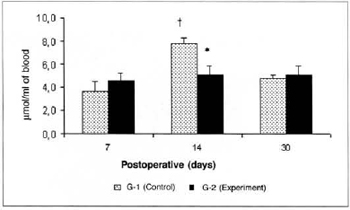
Fig. 7 - Lactate Concentrations in blood (µmol/ml of blood). *(p< 0.0l) when compared to the respective control. †(p< 0.05) when compared to the 7th day in the control graup.
Fig. 8 - Concentrations of lactate in the myocutaneous flap (µmol/g of fresh tissue).
There was a significant reduction (0.325 ± 0.06 versus 0.725 ± 0.07) in the concentrations of ketone bodies in the blood of the animals of group G-2 in relation to the concentrations determined in group G-1 on the 14th post-operative day. We also observed a significant decrease (0.325 ± 0.06 versus 0.516 ± 0.054) in the concentrations of ketone bodies in the blood of the animals of group G-2 on the 14th day when compared to the concentrations determined on the 7th day in the same group (Fig. 9). We also observed a significant increase (0.525 ± 0.10 versus 0.164 ± 0.04) in the concentrations of ketone bodies in the myocutaneous flap (Fig. 10) on the 30th day in relation to the concentrations calculated on the 7th post-operative day in the animals treated (G-2).
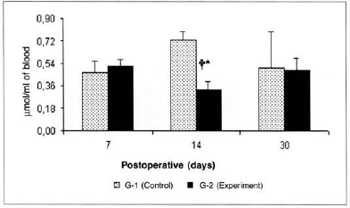
Fig. 9 - Ketone body concentrations in blood (µmol/ml of blood).*(p< 0.05) when compared to the respective control. †(p< 0.05) when compared to the 7th day in the control group.
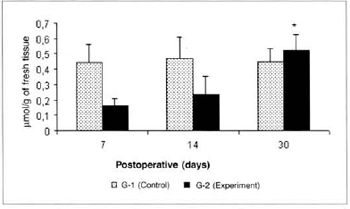
Fig. 10 - Ketone body concentrations in skin (µmol/g of fresh tissue). *(p< 0.05) when compared to the 7th day in the experiment group.
DISCUSSION
The significant drop in the blood concentrations of pyruvate, lactate and ketone bodies on the 14th postoperative day, during the full fibroblastic proliferative phase of the scarring process in the animals treated with L-Arginine food supplementation may be explained by the anabolic action of this amino-acid, which secretes growth hormone and induces the release of insulin and IGF-1. A similar insulin growth factor is present in the liver, plasma and fibroblasts and stimulates the synthesis of sulphated proteoglycans, collagen, and the proliferation of fibroblasts.
The possible elevation in blood insulin would mean a reduction in cytogenesis for the liver, which would explain the decrease in the concentrations of ketone bodies in the blood. Likewise, the anabolic action of these factors would lead to higher uptake and utilization of pyruvate and lactate by peripheral tissues, with consequent drop in the concentrations of these metabolites in the blood.
We know that the NO-synthetase that results from surgical stress helps the synthesis of a specific sequence nitric acid (NO) from the Arginine guanidine radical, acting as an inhibitor of thrombosis and red blood cell aggregation, conditioning the increase of the blood flow in healing tissues. Better oxygenation in the surgical wound site enables epithelialization and increase in the scar tensile strength through the formation of a more stable collagen, helping chemotaxy and thereby facilitating the scarring process(12,13,14).
The significant increase in the concentrations of ketone bodies in the myocutaneous flap on the 30th postoperative day in relation to the amounts determined on the 7th day, in treated animals (G-2), may be explained by a higher anabolism in recipient exogenous L-Arginine animals, with higher uptake and utilization of this metabolite by healing tissues.
The results suggest that the L-Arginine food supplementation, because of its anabolic action, would have some effect on the concentrations of substrates and on skin and muscle scarring.
CONCLUSIONS
1. L-Arginine food supplementation leads to significant reduction in blood concentrations of pyruvate, lactate and ketone bodies, on the 14th post-operative day after the myocutaneous flap procedure.
2. L-Arginine food supplementation induces significant increase in tissue concentrations of ketone bodies on the 30th day post-operative in a healing myocutaneous flap.
REFERENCES
1. Biondo-Simões MLP.Cicatrização.In: Castro e Silva Jr O. Modelos experimentais de pesquisa em cirurgia. São Paulo: Robe; 1998. p.265-74.
2. Modolin MLA, Bevilacqua RG, Margarido NF, Gonçalves EL. Cicatrização das feridas abertas na desnutrição com hipoproteinemia. Estudo experimental. Rev Hosp Clín Fac Med S Paulo. 1982;37:275-8.
3. Polk Jr HC, Lopez-Mayor JF. Postoperative wound infection: a prospective study of determinant factors and prevention. Surgery. 1969;66:103.
4. Corsi RCC, Corsi PR, Pirana S, Muraco FAE, Jorge D. Cicatrização das feridas - Revisão da literatura. Rev Bras Cir. 1994;84:19-53.
5. Peacock EE. Repair and regeneration. In: Reconstructive Plastic Surgery. 2.ed. New York: WB Saunders; 1977. p.78-103.
6. Seifter E, Rettura G, Barbul A, Levenson SM. Arginine: an essential amino acid for injured rats. Surgery. 1978;84(2):224-30.
7. Barbul A, Lazarou SA, Efron DT, Wasserkrug HL, Efron G. Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery. 1990;108:331-7.
8. Silva LFG, Cavalcante JLBG, Soares FSD, Moraes MO, Vasconcelos PRL. Effects of arginine-enriched enteral nutrition in Walker tumor bearing rats in the kidney. Braz J Urol. 2001;27(2):178-85.
9. Hohorst apud Vasconcelos PRL. Hepatic metabolism during sepsis. 1987, 55f. Thesis (Ph.D.) University of Oxford.
10. Williamson DH, Mellanby J, Krebs HA. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962;15:90-6.
11. Slein apud Vasconcelos PRL. Hepatic metabolism during sepsis. 1987, 54f. Thesis (Ph.D.) University of Oxford.
12. Modolin MLA, Bevilacqua RG. Cicatrização das feridas. Síntese das aquisições recentes. Rev Bras Clín Terap. 1985;14(6):208.
13. Faintuch J, Faintuch J. Efeitos cardiovasculares da arginina e do óxido nítrico. Rev Hosp Clín Fac Med S Paulo. 1995;50:334-8.
14. Cohen K, Digelmann LR, Yager D R, Wornum ILL, Graham MF, Croffland MC. Wound care and wound healing. In: Schwartz SI. Principies of surgery 7. ed. New York: Mcgraw-Hill; 1999. p. 263-95.
I - Member of the Brazilian Society of Plastic Surgery Masters student, Stricto Sensu Postgraduate Program in Surgery, Department of Surgery, UFC
III - Fellow of the American College of Surgeons, Master in Surgery from the Federal Universiry of Ceará. Assistant professor of the School of Medicine (UFC)
III - Master in Pharmacology from the Federal Universiry of Ceará
IV - Students, School of Medicine, Federal Universiry of Ceará
V - Ph.D. Professor, Coordinator of the Stricto Sensu Postgraduate Course in Surgery of the School of Medicine (UFC), Fortaleza, CE
Study performed at the Laboratory of Experimental Surgery (LABCEX), of the Department of Surgery, Stricto Sensu Postgraduate Program in Surgery, School of Medicine of the Federal University of Ceará, (UFC), Brazil
Address for correspondence:
Paulo Roberto Leitão de Vasconcelos, MD
Depto. de Cirurgia
R. Prof. Costa Mendes, 1608 - 3° ando
60430-140 - Fortaleza - Ceará Brazil
Phone: (55 85) 288-8063 - Fax: (55 85) 288-8064
e-mail: mcirur@npd.ufe.br


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter