

Case Report - Year 2016 - Volume 31 -
The Gorlin-Goltz Syndrome: diagnosis of a case associated with heart disease and type 2 diabetes mellitus
Síndrome de Gorlin-Goltz: diagnóstico de um caso associado à cardiopatia e diabetes mellitus 2
ABSTRACT
The Gorlin-Goltz syndrome (GGS) is a hereditary, autosomal dominant condition, with high penetrance and variable expressivity, resulting from mutations in the genes PTCH1, PTCH2, or SUFU. The diagnosis is based on the presence of 2 major criteria or a major criterion associated with 2 minor criteria, including multiple basal cell carcinomas, keratocystic odontogenic tumor (KOT), and bifid rib. Other endocrine, neurological, ophthalmologic, genital, respiratory, and cardiovascular alterations are found in the literature, but with variable manifestations. This study reports the case of a patient diagnosed with GGS associated with diastolic congestive heart failure and type 2 diabetes mellitus, who underwent multiple treatments for components of the syndrome. More recently, the patient underwent decompression followed by cystic enucleation of two KOTs in the jaw, oral rehabilitation with removable prosthodontics, cardiological evaluation, and attempted clinical control of endocrine and cardiac problems.
Keywords: Basal cell nevus syndrome; Hypertelorism; Macrocephaly; Buccal pathology; Hypertrophic cardiomyopathy.
RESUMO
A síndrome de Gorlin-Goltz (SGG) é uma condição hereditária, autossômica dominante, com alta penetrância e expressividade variável, decorrente de mutações nos genes PTCH1, PTCH2 ou SUFU. O diagnóstico é baseado na presença de dois critérios maiores ou um critério maior associado a dois critérios menores, dentre eles múltiplos carcinomas basocelulares, tumor odontogênico ceratocístico (TOC) e costela bífida. Outras alterações endócrinas, neurológicas, oftalmológicas, genitais, respiratórias e cardiovasculares são encontradas na literatura, porém com manifestações variáveis. O objetivo deste trabalho é relatar um caso clínico de uma paciente sistematicamente diagnosticada com SGG associada à insuficiência cardíaca congestiva diastólica e diabetes mellitus 2 submetida a tratamentos seriados das respectivas manifestações sindrômicas. Mais recentemente, à descompressão cística seguida da enucleação de dois TOC em mandíbula, reabilitação oral com prótese total removível, avaliação cardiológica e tentativa de controle clínico das alterações endócrinas e cardíacas.
Palavras-chave: Síndrome do nevo basocelular; Hipertelorismo; Macrocefalia; Patologia bucal; Cardiomiopatia hipertrófica.
Initially reported by Jarish and White in 1894, the Gorlin-Goltz syndrome (GGS) was described in 19601. It is a hereditary, autosomal dominant syndrome, with high penetrance and variable expressivity, resulting from mutations in the genes PTCH1, PTCH2 or SUFU, which are located on chromosomes 9q22.32, 1p34.1, and 10q24.32, respectively3, and is registered in Online Mendelian Inheritance in Man (OMIM) under number #109400 as basal cell nevus syndrome (BCNS)3.
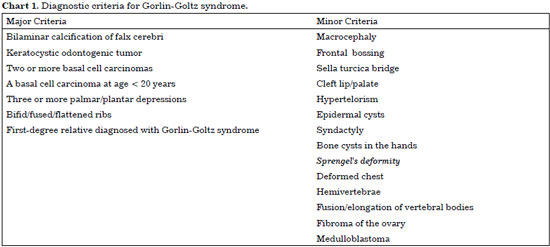
GGS is characterized clinically by multiple basal cell carcinomas (BCC), jaw cysts, and bifid rib1. The prevalence reported in the literature is variably estimated at 1:57,000 or 1:164,000 individuals4. In 1997, it was proposed that the diagnosis of GGS should be based on the presence of 2 major criteria or 1 major criterion and 2 minor criteria5, represented in Chart 1.
Other endocrine, neurological, ophthalmologic, and genital alterations have been reported5, and rare manifestations involve the respiratory and cardiovascular systems6.
Cardiac fibromas are reported in 3-5% of GGS cases6. These tumors should be investigated when irregular cardiac silhouette, cardiomegaly, calcifications after myocardial infarction, or unexplained heart failure are observed7. The most common complications are arrhythmias, conduction disturbances, and congestive heart failure8, with cardiac arrest and ventricular tachycardia reported in the literature9. Transthoracic echocardiography is very useful for tumor localization and analysis of its effect on cardiac function8.
OBJECTIVE
This study reports the case of a patient diagnosed with GGS associated with diastolic congestive heart failure (CHF) and type 2 diabetes mellitus (DM2), who underwent serial treatments for syndrome manifestations. More recently, the patient underwent decompression followed by cystic enucleation of 2 keratocystic odontogenic tumors (KOT) in the jaw, oral rehabilitation with removable complete dentures, and cardiological evaluation and attempted clinical control of endocrine and cardiac abnormalities.
CASE REPORT AND DISCUSSION
CGF patient, female, 59 years old, black, retired due to disability, signed the Free Consent agreement (Figures 1A and 1B). Diagnosed with diastolic CHF, hypertension (HBP) and DM2; using metformin hydrochloride 850 mg, glibenclamide 15 mg, amlodipine 5 mg, hydrochlorothiazide 25 mg, acetylsalicylic acid 100 mg, enalapril 20 mg, and simvastatin 40 mg.

Figure 1. A: Frontal headgear view; B: Side headgear view.
Previous History
Oophorectomy and salpingectomy 38 years prior; excision of 2 facial epidermal cysts over 20 years prior; enucleation of 2 KOTs in the left anterior and posterior mandibular region 4 years prior.
Physical Examination
Height 163 cm, weight 75 kg, body mass index 28.23 kg/m2, controlled blood pressure, head circumference 61 cm, medial intercanthal distance 47 mm, interpupillary distance 77 mm, epidermal cysts in facial region, totally edentulous, undergoing oral rehabilitation.
Supplemental Exams
Fasting glucose 298 mg/dL, glycated hemoglobin 10%, low-density lipoprotein above target; posteroanterior radiograph of face: interorbital distance (IOD) 35 mm, calcification of the falx cerebri (Figure 2); panoramic radiographs: KOT preoperative and postoperative views (Figures 3 and 4); chest X-ray: costal facets without changes, increased cardiothoracic index, pulmonary congestion, and right interlobular edema; EKG: sinus rhythm + left bundle branch conduction disorder and lateral repolarization; transthoracic echocardiography: mild left ventricular concentric hypertrophy, borderline systolic function, 56% ejection fraction, and diastolic dysfunction grade I.

Figure 2. Frontal teleradiography: ocular hypertelorism and calcification of the falx.

Figure 3. Panoramic X-ray: two keratocystic odontogenic tumors in the jaw.

Figure 4. X-ray: after decompression and cystic enucleation.
After analyzing the data from the history, physical examination, and laboratory tests, it was possible to diagnose GGS with cardiac fibroma but not severe cardiovascular manifestations.
The face must be examined in the frontal view to assess bilateral symmetry, midline structural proportions, and lateral and vertical proportionality. The examination of the eyes and the orbits begins with measurement of the intercanthal and interpupillary distances.
The average value of this difference is 4 mm; black people often have greater values10. Ocular hypertelorism is classified into 3 degrees according to the IOD: grade I (30-34 mm), grade II (34-39 mm), and grade III (40 mm or more), after alignment in frontal teleradiography11.
The measurement of head circumference (HC) in adults is important for research and clinical evaluation, and the following formula was proposed to estimate the HC based on height and weight of the patient: 0.063 × Height + 0.040 × Weight + 42.618. Macrocephaly should be considered for values above 90th percentile12.
HC (61 cm) was > 97th percentile, according to the weight and height of the patient12. A 30-mm difference between the intercanthal and interpupillary distances highlights telecanthus; however, ocular hypertelorism was diagnosed and classified by the IOD (35 mm) measured in chest and frontal views, as reported in the literature11.
Epidermal cysts are common skin lesions that occur primarily on the face and upper torso. Pilonidal and sebaceous cysts should be part of the differential diagnosis; dermatitis, lipoma, and basal cell carcinoma may be present13. KOT occurs in up to 75% of patients with GGS14, with distinctive clinical behavior and histological aspects; the jaw is affected in 60-80% of cases.
Large lesions are associated with pain, swelling, and drainage, with growth in anteroposterior direction through medullary spaces. Radiographically, the lesions display a well-defined, peak-like radiolucent area and cortical margin. Histopathological confirmation is required for diagnosis, and treatment includes enucleation or curettage, with recurrence rates of 5-62%2.
The patient previously was found to have ovarian tumors, epidermal cysts, and striking facial features (Figures 1A and 1B), but without a previous diagnosis of GGS. Mandibular cystic lesions were treated by decompression and enucleation with topical application of Carnoy's solution, aiming to reduce surgical morbidity and the risk of relapse, and still preserve mandibular anatomy. Histopathological diagnosis of KOT was followed by examination of the cephalic perimeter, facial analysis, and frontal teleradiography, which enabled confirmation of the diagnosis.
GGS diagnosis may be determined by the presence of 2 major criteria or by the presence of a major criterion and 2 minor criteria: in this case, calcification of the falx cerebri (Figure 2) and the presence of 2 successfully treated KOTs in the jaw with confirmed histopathological features (Figures 3 and 4) were major criteria. Minor criteria in this patient included macrocephaly, hypertelorism grade II (Figure 2), epidermal cysts, and history of oophorectomy and salpingectomy, which suggests a previous diagnosis of ovarian tumor.
Although the life expectancy of GGS patients is not significantly affected, the manifestations are associated with increased morbidity, and medulloblastoma may be present in up to 10% of cases15. Endocrine abnormalities have been reported in the literature5, and this patient had type 2 diabetes mellitus with sustained hyperglycemia, even with the use of an oral hypoglycemic agent.
The only cardiac alterations found in the literature associated with GGS are cardiac fibromas6,7 and arrythmias8,9, including ventricular tachycardia with right bundle branch block as a consequence of this tumor9. Even though the patient had diastolic CHF, a hypertensive etiology would be more plausible, as suggested by the literature, due to its correlation with GGS.
The clinical condition of the patient required attention, especially with regard to cardiovascular risk factors and treatment of possible cardiac decompensation, present as pulmonary congestion; a loop diuretic (furosemide 40 mg) was prescribed, the dose of the statin was increased to 40 mg, and endocrinology consultation was requested.
Thus, because of involvement of multiple organs and systems, it is essential that such patients receive multidisciplinary evaluation, including care from the medical clinic, orthopedics, dermatology, neurology, ophthalmology, and gynecology or urology, in addition to genetic counseling16.
GGS presents well-established diagnostic criteria, but requires specific diagnostic information. Craniofacial, skeletal, and skin abnormalities, as well as KOT were the main focus of attention. Endocrine, neurological, ophthalmic, respiratory, and genital changes may be present; however, further studies are needed to determine the correlation between GGS and congenital heart defects other than cardiac fibroma.
Integrated care optimizes prognosis and the patient's quality of life.
COLLABORATIONS
JPND Analysis and/or interpretation of the data; conception and design of the study; performance of operations and/or experiments; writing of the manuscript or critical review of its contents.
BBA Analysis and/or interpretation of data.
WRS Final approval of the manuscript.
FMMO Final approval of the manuscript.
REFERENCES
1. Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960;262:908-12.
2. Neville WB, Damm DD, Allen CM, Bouqout JE. Patologia Oral e Maxilofacial. 1a ed. Rio de Janeiro: Guanabara Koogan; 1998. p.485-90.
3. McKusick VA, O´Neill MJF. OMIM© [Internet]. Basal Cell Nevus Syndrome; BCNS. Baltimore: John Hopkins University; 1986. [Update 2016 Jul 9] [citado 2016 Jul 27]. Disponível em: http://omim.org/entry/109400
4. Manfredi M, Vescovi P, Bonanini M, Porter S. Nevoid basal cell carcinoma syndrome: a review of the literature. Int J Oral Maxillofac Surg. 2004;33(2):117-24.
5. Pandeshwar P, Jayanthi K, Manesh D. Gorlin-goltz syndrome. Case Rep Dent. 2012;2012:247239.
6. Evans DG, Ladusans EJ, Rimmer S, Burnell LD, Thakker N, Farndon PA. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet. 1993;30(6):460-4.
7. Bossert T, Walther T, Vondrys D, Gummert JF, Kostelka M, Mohr FW. Cardiac fibroma as an inherited manifestation of nevoid basal-cell carcinoma syndrome. Tex Heart Inst J. 2006;33(1):88-90.
8. Coffin CM. Congenital cardiac fibroma associated with Gorlin syndrome. Pediatr Pathol. 1992;12(2):255-62.
9. Nakamura T, Miyazawa H, Ishida T, Shimada Y. Ventricular tachycardia in a patient with Gorlin syndrome. Intern Med. 2013;52(7):831-2.
10. Suguino R, Ramos AL, Terada HH, Furquim LZ, Maeda L, Silva Filho OG. Análise facial. Rev Dental Press Ortod Ortop Maxilar. 1996;1(1):86-107.
11. Amaral CER, Amaral CAR, Bradley JP, Guidi MC, Buzzo CL. Estudo da recidiva após correção do hiperteleorbitismo. Rev Bras Cir Plást. 2009;24(4):425-31.
12. Nguyen AKD, Simard-Meilleur AA, Berthiaume C, Godbout R, Mottron L. Head Circumference in canadian male adults: development of a normalized chart. Int J Morphol. 2012;30(4):1474-80.
13. Alves JR, Hida M, Nai GA. Diagnóstico clínico e anatomopatológico: discordâncias. Rev Assoc Med Bras. 2004;50(2):178-81.
14. Díaz-Fernández JM, Infante-Cossío P, Belmonte-Caro R, Ruiz-Laza L, García-Perla-García A, Gutiérrez-Pérez JL. Basal cell nevus syndrome. Presentation of six cases and literature review. Med Oral Patol Oral Cir Bucal. 2005;10 Suppl 1:E57-66.
15. Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Orphanet J Rare Diseases. 2008;3:32.
16. Souza-e-Souza I, Cunha PCAS. Síndrome de Goltz: relato de dois casos. An Bras Dermatol. 2003;78(1):91-7.
1. Faculdade de Medicina, Universidade de Santo Amaro, São Paulo, SP, Brazil
2. Programa de Pós-Graduação em Odontologia, Universidade de Santo Amaro, São Paulo, SP, Brazil
3. Departamento de Radiologia e Diagnóstico por Imagem, Faculdade de Ciências Médicas da Santa Casa de São Paulo, São Paulo, SP, Brazil
Institution: Universidade de Santo Amaro, São Paulo, SP, Brazil.
Corresponding author:
João Paulo Nunes Drumond
Rua Prof. Enéas de Siqueira Neto, 340 - Jardim das Imbuias
São Paulo, SP, Brazil Zip Code 04829-300
E-mail: jpnd@uai.com.br
Article received: July 29, 2016.
Article accepted: October 30, 2016.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter