

Original Article - Year 2017 - Volume 32 -
Skull reconstruction with PMMA customized prostheses after decompressive craniectomies
Reconstrução de calota craniana com prótese customizada de PMMA após craniectomias descompressivas
ABSTRACT
INTRODUCTION: Decompressive craniectomy is indicated for the treatment of intracranial hypertension in cases of serious traumatic brain injury. This surgery results in a bizarre appearance, as if "part of the head" had been. After regression of cerebral edema and when the patient is in good clinical condition, the reconstruction of the skull is indicated. Reconstruction of the skull can be performed with autologous bone or with alloplastic materials. This study sought to present the experience of the author with skull reconstructions using custom PMMA prostheses.
METHODS: In between 2014 and 2015, fourteen patients with previous (longer than 6 months) decompressive craniectomies were selected after Neurosurgery medical clearance and underwent skull reconstruction with customized PMMA prototyped prostheses. Signs and symptoms of syndrome of the trephined, computed tomography, and aesthetic appearance of the patients were analyzed preoperatively and at 6 months after reconstruction.
RESULTS: All patients presented with improved symptomatology, aesthetic improvement and expansion of the brain after surgery.
CONCLUSION: Reconstruction of the skull with customized prototyped PMMA prostheses improved the signs and symptoms and the aesthetic appearance in all 14 patients of this series. The use of prototypes to customize cranial prostheses facilitates the operative technique and enables patients to develop a nearly normal cranial contour.
Keywords: PMMA; Intracranial hypertension; Decompressive craniectomy; Craniocerebral injuries; Prostheses and implants; Aesthetics.
RESUMO
INTRODUÇÃO: A craniectomia descompressiva é uma cirurgia indicada no tratamento da hipertensão intracraniana em situações graves de traumas cranioencefálicos. Esta cirurgia confere uma aparência bizarra ao paciente, como se "parte da cabeça" houvesse sido retirada. Após a regressão do edema cerebral e quando o paciente reunir boas condições clínicas, a reconstrução craniana está indicada. A reconstrução da calota craniana poderá ser realizada com osso autólogo ou com materiais aloplásticos. Este estudo objetiva apresentar a experiência do autor com reconstruções de calota craniana utilizando próteses customizadas de PMMA.
MÉTODOS: Foram selecionados 14 pacientes submetidos à craniectomia descompressiva que, após serem liberados clinicamente pela Neurocirurgia, realizaram a reconstrução da calota craniana com próteses de PMMA customizadas por prototipagem entre os anos de 2014 e 2015 e com, no mínimo, 6 meses de pós-operatório. Sinais e sintomas de síndrome do Trefinado, tomografia computadorizada e aparência estética dos pacientes foram analisadas no pré e no 6º mês pós-operatório.
RESULTADOS: Todos os pacientes apresentaram melhora sintomatológica, melhora estética e expansão cerebral após a cirurgia.
CONCLUSÃO: A reconstrução da calota craniana com prótese customizada de PMMA promoveu a melhora dos sinais e sintomas e da aparência estética de todos os 14 pacientes operados. A utilização de protótipos para customizar próteses cranianas facilitou a técnica operatória e possibilitou a recuperação de um contorno craniano muito próximo da normalidade.
Palavras-chave: PMMA; Hipertensão intracraniana; Craniectomia descompressiva; Traumatismos craniocerebrais; Próteses e implantes; Estética.
Decompressive craniectomy is indicated for the treatment of intracranial hypertension in severe situations of traumatic brain injury1-4. This procedure consists of the monobloc removal of a large part of the frontal, temporal, parietal and/or sphenoid bones of the affected side, thereby allowing the free expansion of cerebral edema without the limitations of the cranial vault. Although this procedure saves lives in many cases, it confers a bizarre appearance to the patient, as if "part of the head" had been removed.
After regression of cerebral edema and when the patient is in good clinical condition, skull reconstruction is indicated5. The surgery aims to reacquire cerebral protection against trauma, recover the cranial contour, and improve the neurological symptoms with the reestablishment of physiological intracranial pressure. Restoration of the anatomic barrier between intracranial structures and the environment normalizes cerebrospinal fluid and cerebral blood flow dynamics. The set of signs and symptoms resulting from the partial loss of the skull is called syndrome of the trephined6-11.
The reconstruction of the skull can be performed with autologous bone or with alloplastic materials12,13. Autologous bone has a greater resistance to infection and a lower probability of extrusion; however, it may suffer from variable absorption, be difficult to model, and increase morbidity in the donor5,6,14-16.
Grafting of the parietal bone is the first choice whenever possible. In the case of reconstruction after decompressive craniectomy, the size of the defect practically impedes this option due to the lack of donor area. Alloplastic materials offer an excellent contour, but there is a higher risk of infection and extrusion. The most commonly used alloplastic materials are polymethyl methacrylate (PMMA), hydroxyapatite (HA), and titanium14,17-19.
PMMA has been used since 1940 by Zander (apud Sanan and Haines20) to repair craniofacial defects and is the choice of many authors21-24. In Brazil, it is the alloplastic material that is most often available in the Brazilian public health system (SUS) for skull reconstruction. It consists of a kit with a polymer powder component (30g) and a liquid monomer component (17 ml), which, when mixed, forms an acrylic resin in a polymerization process20. During polymerization, the PMMA will harden gradually and can be shaped in a way that fits to the bone defect.
The molding of the PMMA can be performed in the pre-operative or intraoperative time and it can be either modeled manually on the defect25,26, manually with the help of templates27-30, or by a 3D printer using prototyping. The printing of customized prostheses for prototyping is an excellent alternative for this type of reconstruction, given its benefits in accuracy of the cranial contour and the facilitation of operative technique, among other advantages31,32. Unfortunately, the cost of custom cranial prostheses, of whichever material, currently is prohibitive in the Brazilian public health system.
The Hospital da Restauração (HR), in Recife, PE, is the regional referral center for cranial trauma and its sequelae. Reconstruction of the skull in patients with previous decompressive craniectomy had been performed with intraopeartive manual molding of PMMA into bone defect. Since 2014, the Department of Plastic Surgery, HR has performed these reconstructions with customized PMMA prototyped prostheses.
OBJECTIVE
This study sought to share the experiences of the authors in reconstructions of the skull using custom PMMA prototyped prostheses. This scientific publication was used to obtain the title of Titular Member of the Brazilian Society of Plastic Surgery.
METHODS
This is a prospective interventional, descriptive study carried out at the Hospital da Restauração (HR), in Recife, PE, by the Plastic Surgery and Neurosurgery services. Fourteen patients who underwent decompressive craniectomy, were cleared clinically by the Neurosurgery service, underwent reconstruction of the skullcap with customized PMMA prototyped prostheses between 2014 and 2015. Reconstruction was performed at least 6 months after craniectomy procedure. All patients provided written informed consent and the study protocol followed the principles outlined in the Declaration of Helsinki.
A questionnaire with the signs and symptoms of the syndrome of the trephined and evaluation by computerized tomography (CT) pre-operatively and at 6 months after surgery were performed by the service of Neurosurgery. The signs and symptoms assessed were: local discomfort, headache, dizziness, tinnitus, insomnia, fatigue, irritability, depression, insecurity, intolerance to vibration, convulsions, paresis, dysphasia, dyspraxia, attention deficit, memory deficit, and worsening of symptoms upon standing or during the Valsalva maneuver. In addition, postoperative outcomes and complications were analyzed.
In the 6th month post-surgery, patients also answered questions regarding the aesthetic result of surgery. This assessment asked patients if the cranial contour was satisfactory or unsatisfactory and requested an assessment in grades from 1 to 5. The scores from 1 to 5 represented very poor (1), poor (2), good (3), very good (4) and excellent (5).
The age group ranged from 18 to 54 years, with the mean age being 31 years of age. All patients were males who experienced a head injury, which prompted the decompressive craniectomy, and none had previously undergone any attempt of reconstruction of the skull. The bone defects resulting from decompressive craniectomy are described in Table 1.
Patients with bone defects underwent a CT scan (Somatom Definition AS 64-slice, Siemens®) with slices ≤ 1 mm. The examinations were recorded as a file in DICOM format on a DVD (Figure 1). The DVDs were sent to Renato Archer Information Technology Center (CTI RA) in Campinas, SP. The images of the computed tomography scans were loaded in the InVesalius® software to develop the prototypes and subsequently printed using a 3D printer (SLS HiQ, 3D System®). In the cases of reconstruction of the skull, the following were developed:
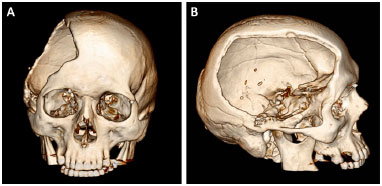
Figure 1. A and B: Computed tomography of the skull of a patient with a defect resulting from a decompressive craniectomy.
• Prototype 1: defective skull (Figure 2).
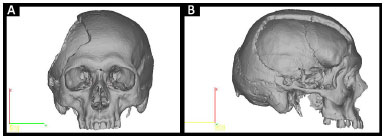
Figure 2. A and B: Planning prototype 1 by InVesalius®: faulty Skull.
• Prototype 2: a piece that is missing from the skull defect (Figure 3).
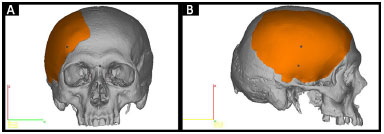
Figure 3. A and B: Planning prototype 2 by InVesalius®: piece that is missing in the defective skull (in orange).
• Prototype 3: two molds (two pieces) that allow the construction of a perfect copy of Prototype 2 (Figure 4).
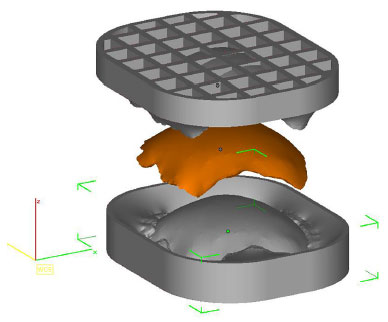
Figure 4. Planning of two forms by InVesalius® that enables a copy of prototype 2 (in orange).
The prototypes are printed using polyamide (PA12) plastic material sintering technology and, therefore, are not biocompatible and cannot be implanted in humans. Hence, developing prototype 3 makes it possible to manufacture the cranial prosthesis in biocompatible material during the intraoperative period. All prototypes are sterilized in a steam autoclave at 134ºC for 5 minutes and taken to the operating room (Figure 5).
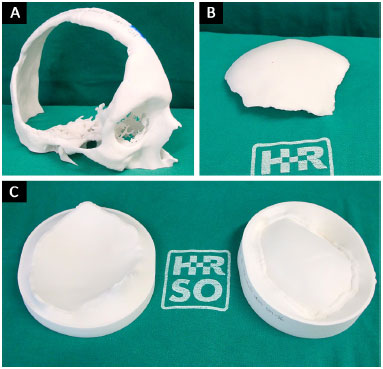
Figure 5. A, B and C: Printed and sterile prototypes 1, 2 and 3 in the surgery room.
While the patients are under general anesthesia and undergoing an antisepsis regimen with chlorhexidine degermant and alcohol, the custom prosthesis is constructed with surgical PMMA cement (Subiton Cranioplastias® or Codman Cranioplastic®) (Figure 6). The powder of the product is mixed with the liquid portion in a vat until it is ready to model, which occurs in a few minutes and is indicated when the material no longer adheres to gloves.
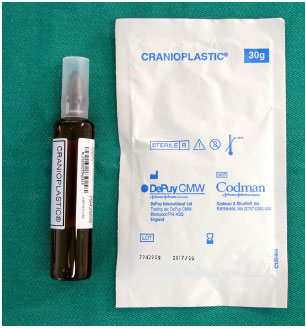
Figure 6. PMMA surgical cement for cranioplasty: powder component and liquid component.
At that moment, the cement is placed in the prototyped molds, which are then closed. After a few minutes, the cement hardens and the prosthesis is removed (Figure 7). With the prosthesis already constructed and using prototype 1, we can verify its fit, make possible adjustments, position it correctly, and bend and fix the plates on the prosthesis (Figure 8).
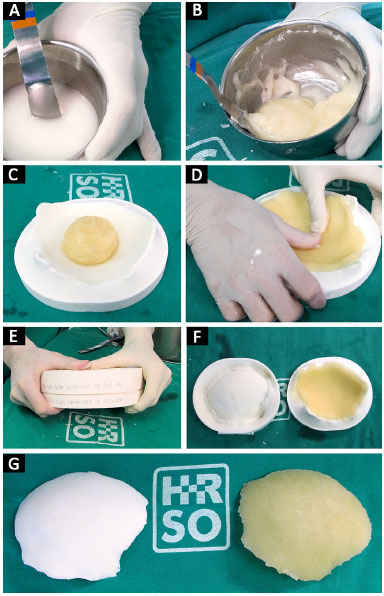
Figure 7. A, B, C, D, E, F and G: Polymerization of PMMA from liquid to solid; modeling of PMMA on the mold; closure of molds manufactured prosthesis.
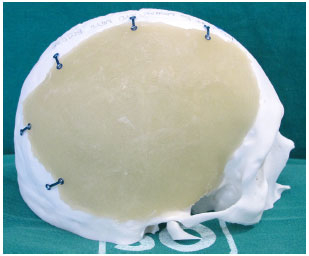
Figure 8. Prosthesis adjusted and positioned, with the plates folded and fastened with screws.
Surgical access is performed through previous scar from decompressive craniectomy without resection of the scar edges prevent tension upon closure (Figure 9). The defect is then exposed by elevating the scalp on the plane just above the dura mater, while leaving the coverage as thick as possible. In the temporal region, the temporal muscle is also elevated from the dura mater (Figure 10).
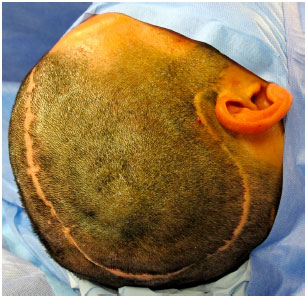
Figure 9. Surgical access on a prior scar of the decompressive craniectomy without resection of scar edges.
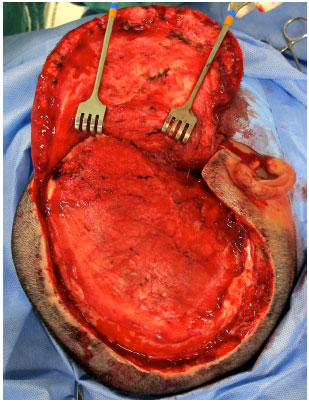
Figure 10. Defect exposed in the plane just above the dura mater.
The prosthesis is then inserted into the defect and fixed with plates and titanium screws (Bioplate®, 1.6 System with 2 holes per plate) (Figure 11). A silicone tubular continuous suction drain is positioned and the wound is closed in the galeal plane with Capofril® 2-0 and in the cutaneous plane with Mononylon® 2-0 in separate sutures. After surgery, the patient is admitted to the Unit of Advanced Support of Neurosurgery (USAN), where a control tomography is performed in the first 12 hours. The drain is removed when the output is less than 50 ml within 12 hours and hospital discharge usually occurs within 48 hours.
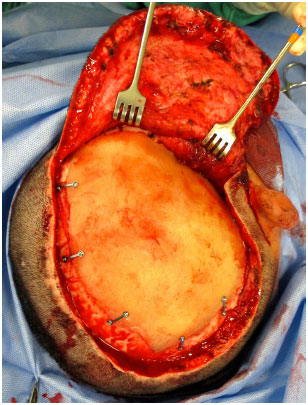
Figure 11. Customized PMMA skull prosthesis already fixed on the defect with plates and screws.
RESULTS
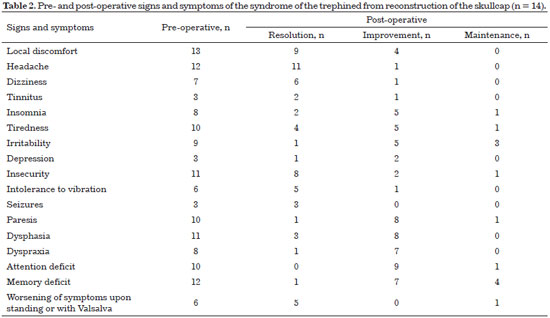
All patients exhibited improved symptomatology after reconstruction of the skull (Table 2).
Head CT scans were performed 6 months after reconstruction, which indicated that cerebral expansion occurred in all patients (Figure 12). Exceptionally, one of the tests was performed 2 months after reconstruction. The patient in this test also exhibited brain expansion.
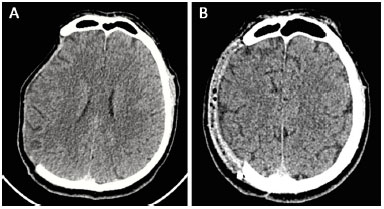
Figure 12. A and B: Computed tomography performed pre-operatively and at 6 months after surgery showed cerebral expansion after the reconstruction of the skull with a prosthesis.
All 14 reconstructions produced a satisfactory aesthetic result. Eleven patients found the result excellent and three patients classified the result as very good. No patient classified the aesthetic result as very poor, poor, or good (Figures 13 and 14).
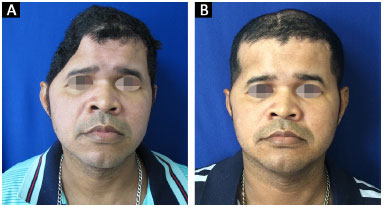
Figure 13. A and B: Patient 10 preoperatively and at 6 months after surgery.
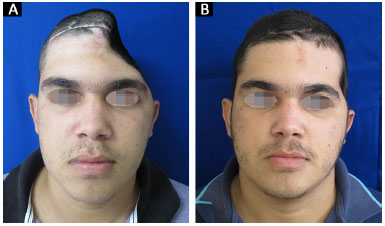
Figure 14. A and B: Patient 14 preoperatively and at 6 months after surgery.
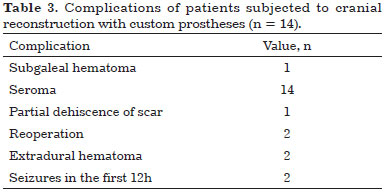
All patients presented seroma postoperatively and were treated with office needle aspiration (twice a week) and compressive dressings. There were no cases of infection.
Two patients presented extradural hematoma: one asymptomatic epidural hematoma on the 4th postoperative day (POD) and other symptomatic on the 10th POD after seroma aspiration. Both were reoperated and the prostheses were relocated.
Two patients had seizures within the first 12 hours postoperatively. These patients were treated with intravenous anticonvulsants and urgent head CT evaluation did not show any abnormality. There were no neurological sequelae in any of the patients (Table 3).
DISCUSSION
The use of customized prototyped prostheses in skull reconstruction has several advantages, including its ability to facilitate the surgical technique31,32 and the excellent cranial contour that is acquired by the prostheses. Since 2014, with the help of the CTI RA, which has been a unit of the Ministry of Science, Technology and Innovation since 1982 and makes prototypes for SUS patients at no cost, the HR Plastic Surgery service started to conduct an alternative method of intraoperative customization with prototyping.
The expense of custom prosthesis can be summed up in two cement units for cranioplasty. Achieving this surgery at an affordable cost enables the SUS patient access to a 1st world technology.
The neurological signs and symptoms of patients with post-decompression craniectomy defects may be secondary to traumatic brain injury or the absence of bone per se. The lack of bone is related to changes in the circulation of cerebrospinal fluid33, to the effect of atmospheric pressure compressing the cortex, and to the reduction of venous return caused by the obliteration of the sub-arachnoid space34. All patients presented improved postoperative symptoms after reconstruction. In 1945, Gardner34 had already testified to the improvement of neurological function after cranioplasty, which was later confirmed by several other authors35-43.
The syndrome of the trephined is also called "Sinking Skin Flap Syndrome,"6-10 precisely because of the depression that is observed in the scalp of the affected side. Frequently, after the placement of the cranial prosthesis, a residual dead space between the dura mater and the inner surface of the prosthesis is observed in the CT from the immediate post-operative period, which can range from millimeters to centimeters.
This dead space, theoretically, can be a facilitating factor of an infectious process that, at the least, will require the immediate removal of the prosthesis. Fortunately, our results showed that the brain expands and occupies the dead space in all cases. In the control CT performed 6 months after the surgery, the brain had expanded in all patients.
This is a surgery with functional gains in which the aesthetic factor has a significant impact on the rehabilitation of patients. Although patients considered the new cranial contour excellent and good, there is almost always a variable asymmetry in the temporal region.
This asymmetry develops in soft tissue, as the prosthesis allows excellent bone symmetry. We believe that this occurs for two reasons: the lack of repositioning of the temporal muscle at the end of the cranial decompression surgery and the atrophy of the same muscle during the time it was disinserted from the temporal fossa. Thus, the temporal muscle tends to be less bulky and "retracts" caudally, creating a bulge above the zygomatic arch in some cases.
In 2014, Reddy et al.44 published a large series of cranioplasties (n = 195) in which infection was reported in 15.9% of cases, seroma in 2.5% of cases, dehiscence in 4.6% of cases, reoperation in 23% of cases, and extrusion of tissue in 11.8% of cases. Compared with our study (n = 14), in which we observed infection in 0% of cases, seroma in 100% of cases, dehiscence in 7% of cases, reoperation in 14% of cases, and extrusion of prostheses in 0% of cases. Notably, we observed seroma and avoided infection in all patients.
The large tissue displacement and the presence of a residual dead space are contributing factors to the formation of seroma. We were diligent with early diagnosis of seroma and aggressive treatment with office needle aspiration twice weekly and compressive dressings until its resolution. Among our patients, three patients presented with seroma until the 2nd month after surgery. Although there were no cases of infection, we had one case of dehiscence from a spontaneous drainage point of the seroma. This dehiscence was resolved with dressings and aspiration of the seroma. The application of quilting sutures between the flap of the scalp and the prosthesis could be a technical artifice that diminishes these high seroma rates.
Even with a custom prosthesis adapting perfectly to the bone defect, there is hardly a complete separation between the extradural space (dura mater - prosthesis) and subgaleal space (prosthesis - scalp flap). Therefore, in the presence of a subgaleal collection, it is possible that there will always be an extradural collection.
This developed in one patient where a subgaleal hematoma could not be evacuated by the surgical drain or by aspiration with a large caliber needle. Furthermore, the CT scan revealed an asymptomatic epidural hematoma with deviation of the middle line. This patient was using valproic acid as an anticonvulsant, which we subsequently discovered alters coagulation.
The other case occurred after a seroma puncture in which there was an inadvertent lesion of a vessel in the temporal region and, similarly, a subgaleal hematoma became a rapidly evolving symptomatic extradural hematoma. Both hematomas were drained surgically, the prostheses were repositioned, and there were no neurological sequelae.
CONCLUSION
Skull reconstruction with a customized PMMA prosthesis promoted the improvement of the symptoms and aesthetic appearance of all 14 patients. The use of prototypes to customize cranial prostheses can facilitate the operative technique and allow patients to develop a normal cranial contour.
COLLABORATIONS
JPBRM Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
ACC Final approval of the manuscript; drafting of the manuscript or critical review of its contents.
REFERENCES
1. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104(4):469-79. PMID: 16619648 DOI: http://dx.doi.org/10.3171/jns.2006.104.4.469
2. Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90(2):187-96. PMID: 9950487 DOI: http://dx.doi.org/10.3171/jns.1999.90.2.0187
3. Honeybul S, Ho KM, Lind CR, Corcoran T, Gillett GR. The retrospective application of a prediction model to patients who have had a decompressive craniectomy for trauma. J Neurotrauma. 2009;26(12):2179-83. DOI: http://dx.doi.org/10.1089/neu.2009.0989
4. Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41(1):84-92. DOI: http://dx.doi.org/10.1097/00006123-199707000-00018
5. Honeybul S, Ho KM. How "successful" is calvarial reconstruction using frozen autologous bone? Plast Reconstr Surg. 2012;130(5):1110-7. PMID: 23096611
6. Baumeister S, Peek A, Friedman A, Levin LS, Marcus JR. Management of postneurosurgical bone flap loss caused by infection. Plast Reconstr Surg. 2008;122(6):195e-208e. PMID: 19050490 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181858eee
7. Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: cosmetic or therapeutic? Surg Neurol. 1997;47(3):238-41. PMID: 9068693
8. Dujovny M, Agner C, Aviles A. Syndrome of the trephined: theory and facts. Crit Rev Neurosurg. 1999;9(5):271-8. DOI: http://dx.doi.org/10.1007/s003290050143
9. Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110(4):488-512. PMID: 17857467 DOI: http://dx.doi.org/10.1097/00000658-193910000-00002
10. Yamaura A, Makino H. Neurological deficits in the presence of the sinking skin flap following decompressive craniectomy. Neurol Med Chir (Tokyo). 1977;17(1 Pt 1):43-53. DOI: http://dx.doi.org/10.2176/nmc.17pt1.43
11. Honeybul S. Decompressive craniectomy: a new complication. J Clin Neurosci. 2009;16(5):727-9. DOI: http://dx.doi.org/10.1016/j.jocn.2008.06.015
12. Aydin S, Kucukyuruk B, Abuzayed B, Aydin S, Sanus GZ. Cranioplasty: Review of materials and techniques. J Neurosci Rural Pract. 2011;2(2):162-7. DOI: http://dx.doi.org/10.4103/0976-3147.83584
13. Manson PN, Crawley WA, Hoopes JE. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986;77(6):888-904. PMID: 3520618 DOI: http://dx.doi.org/10.1097/00006534-198606000-00003
14. Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: a systematic review. Neurosurgery. 2011;68(4):1124-9. DOI: http://dx.doi.org/10.1227/NEU.0b013e31820a5470
15. Cheng YK, Weng HH, Yang JT, Lee MH, Wang TC, Chang CN. Factors affecting graft infection after cranioplasty. J Clin Neurosci. 2008;15(10):1115-9. DOI: http://dx.doi.org/10.1016/j.jocn.2007.09.022
16. Gao LL, Rogers GF, Clune JE, Proctor MR, Meara JG, Mulliken JB, et al. Autologous cranial particulate bone grafting reduces the frequency of osseous defects after cranial expansion. J Craniofac Surg. 2010;21(2):318-22. DOI: http://dx.doi.org/10.1097/SCS.0b013e3181cf5f8b
17. Sahoo N, Roy ID, Desai AP, Gupta V. Comparative evaluation of autogenous calvarial bone graft and alloplastic materials for secondary reconstruction of cranial defects. J Craniofac Surg. 2010;21(1):79-82. DOI: http://dx.doi.org/10.1097/SCS.0b013e3181c3ba58
18. Gosain AK; Plastic Surgery Eductional Foundation DATA Committee. Biomaterials for reconstruction of the cranial vault. Plast Reconstr Surg. 2005;116(2):663-6. PMID: 16079708 DOI: http://dx.doi.org/10.1097/01.prs.0000176289.05374.5b
19. Zins JE, Langevin CJ, Nasir S. Controversies in skull reconstruction. J Craniofac Surg. 2010;21(6):1755-60. DOI: http://dx.doi.org/10.1097/SCS.0b013e3181c34675
20. Sanan A, Haines SJ. Repairing holes in the head: a history of cranioplasty. Neurosurgery. 1997;40(3):588-603. PMID: 9055300
21. Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien). 2006;148(5):535-40. DOI: http://dx.doi.org/10.1007/s00701-006-0740-6
22. Blum KS, Schneider SJ, Rosenthal AD. Methyl methacrylate cranioplasty in children: long-term results. Pediatr Neurosurg. 1997;26(1):33-5. DOI: http://dx.doi.org/10.1159/000121158
23. Marchac D, Greensmith A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J Plast Reconstr Aesthet Surg. 2008;61(7):744-52. DOI: http://dx.doi.org/10.1016/j.bjps.2007.10.055
24. Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg. 2003;14(2):144-53. DOI: http://dx.doi.org/10.1097/00001665-200303000-00003
25. Kanashiro E, Goldenberg DC, Lima DSC, Alonso N, Ferreira MC. Protocol of using methylethacrylate in craniofacial reconstructive surgery. Rev Soc Bras Craniomaxilofac. 2007;10(1):11-8.
26. Bot GM, Ismail NJ, Usman B, Shilong DJ, Obande JO, Aliu S, et al. Using the head as a mould for cranioplasty with methylmethacrylate. J Neurosci Rural Pract. 2013;4(4):471-4. DOI: http://dx.doi.org/10.4103/0976-3147.120207
27. Cerqueira A, Pereira Júnior FB, Azevêdo MS, Ferreira TG. Reconstruction of frontal vault with polimethylmethacrylate implant: a report of two cases. Rev Cir Traumatol Buco-Maxilo-Fac. 2011;11(3):61-8.
28. Caro-Osorio E, De la Garza-Ramos R, Martínez-Sánchez SR, Olazarán-Salinas F. Cranioplasty with polymethylmethacrylate prostheses fabricated by hand using original bone flaps: Technical note and surgical outcomes. Surg Neurol Int. 2013;4:136. DOI: http://dx.doi.org/10.4103/2152-7806.119535
29. Sharavanan GM, Jayabalan S, Rajasukumaran K, Veerasekar G, Sathya G. Cranioplasty using presurgically fabricated presterilised polymethyl methacrylate plate by a simple, cost-effective technique on patients with and without original bone flap: study on 29 patients. J Maxillofac Oral Surg. 2015;14(2):378-85. DOI: http://dx.doi.org/10.1007/s12663-014-0670-4
30. Fernandes da Silva AL, Borba AM, Simão NR, Pedro FL, Borges AH, Miloro M. Customized polymethyl methacrylate implants for the reconstruction of craniofacial osseous defects. Case Rep Surg. 2014;2014:358569. DOI: http://dx.doi.org/10.1155/2014/358569
31. de Farias TP, Dias FL, Galvão MS, Boasquevisque E, Pastl AC, Albuquerque Sousa B. Use of prototyping in preoperative planning for patients with head and neck tumors. Head Neck. 2014;36(12):1773-82. DOI: http://dx.doi.org/10.1002/hed.23540
32. Gerstle TL, Ibrahim AM, Kim PS, Lee BT, Lin SJ. A plastic surgery application in evolution: three-dimensional printing. Plast Reconstr Surg. 2014;133(2):446-51. PMID: 24469175 DOI: http://dx.doi.org/10.1097/01.prs.0000436844.92623.d3
33. Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res. 1997;19(3):311-6. DOI: http://dx.doi.org/10.1080/01616412.1997.11740818
34. Richaud J, Boetto S, Guell A, Lazorthes Y. Effects of cranioplasty on neurological function and cerebral blood flow. Neurochirurgie. 1985;31(3):183-8.
35. Gardner WJ. Closure of defects of the skull with tantalum. Surg Gynecol Obstet. 1945;80:303-12.
36. Grantham EC, Landis HP. Cranioplasty and the posttraumatic syndrome. J Neurosurg. 1948;5(1):19-22. DOI: http://dx.doi.org/10.3171/jns.1948.5.1.0019
37. Agner C, Dujovny M, Gaviria M. Neurocognitive assessment before and after cranioplasty. Acta Neurochir (Wien). 2002;144(10):1033-40. DOI: http://dx.doi.org/10.1007/s00701-002-0996-4
38. Harner SG, Beatty CW, Ebersold MJ. Impact of cranioplasty on headache after acoustic neuroma removal. Neurosurgery. 1995;36(6):1097-9. DOI: http://dx.doi.org/10.1227/00006123-199506000-00005
39. Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53(3):288-92. DOI: http://dx.doi.org/10.1097/01.sap.0000106433.89983.72
40. Schiffer J, Gur R, Nisim U, Pollak L. Symptomatic patients after craniectomy. Surg Neurol. 1997;47(3):231-7. PMID: 9068692 DOI: http://dx.doi.org/10.1016/S0090-3019(96)00376-X
41. Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery. 1994;34(4):729-31. DOI: http://dx.doi.org/10.1227/00006123-199404000-00024
42. Tabaddor K, LaMorgese J. Complication of a large cranial defect. Case report. J Neurosurg. 1976;44(4):506-8. PMID: 1255240 DOI: http://dx.doi.org/10.3171/jns.1976.44.4.0506
43. Yamaura A, Sato M, Meguro K, Nakamura T, Uemura K. Cranioplasty following decompressive craniectomy--analysis of 300 cases (author's transl). No Shinkei Geka. 1977;5(4):345-53.
44. Reddy S, Khalifian S, Flores JM, Bellamy J, Manson PN, Rodriguez ED, et al. Clinical outcomes in cranioplasty: risk factors and choice of reconstructive material. Plast Reconstr Surg. 2014;133(4):864-73. PMID: 24675189 DOI: http://dx.doi.org/10.1097/PRS.0000000000000013
1. Sociedade Brasileira de Cirurgia Plástica, Recife, PE, Brazil
2. Associação Brasileira de Cirurgia Crânio-Maxilo-Facial, São Paulo, SP, Brazil
3. Hospital da Restauração, Recife, PE, Brazil
4. Hospital das Clínicas, Universidade Federal de Pernambuco, Recife, PE, Brazil
5. AVIVA Cirurgia Plástica, Recife, PE, Brazil
Institution: Hospital da Restauração, Recife, PE, Brazil.
Corresponding author:
Pablo Maricevich
AVIVA Cirurgia Plástica AVIVA Cirurgia Plástica
Av. Antônio de Góes, 275, sala 407 - Pina
Recife, PE, Brazil Zip Code 51110-000
E-mail: pablo@avivacirurgiaplástica.com.br
Article received: July 29, 2015.
Article accepted: September 29, 2015.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter