

Articles - Year 2003 - Volume 18 - Issue 2
Vegetable Polyurethane Resin Implant in Cranioplasty - Experimental Study in Rabbits
Implante de Resina Poliuretana Vegetal em Cranioplastia - Estudo Experimental em Coelhos
ABSTRACT
An experimental model was used to evaluate a new biomaterial - a vegetable polyurethane resin, extracted from castor plant oil- for repairing cranial vault bone loss. Two cranial, 15 x 10 mm in total width diameter and periosteum-free, bone failures were performed in 31 adult rabbits on both sides of the parietal region. Failures were not repaired in the 13 animals that comprised the control group and 18 animals had deficts repaired using a vegetable polyurethane resin implant. Macroscopic, radiological and histological assessments were peiformed at 2, 6, 12, 18 and 24 post-operative weeks. Regardless of the method of evaluation, no tissue repair by bone neo-formation could be observed in the control group in which bone failures were replaced by scar tissue. In the group with implants, as of 6 weeks, repair of deficts by bone neogenesis in peripheral areas was observed with a progressive favorable development in late samples (18 and 24 weeks) along with osteogenesis, osteoconduction and osteopromotion. Statistical analyses confirmed that bone healing in the implanted deficts was more significantly effictive (p< 0.05). No toxic or reactional phenomena secondary to the presence of the implants that were not incorporated in the observational period were detected. No osteoinductive proprieties of implants became evident.
Keywords: Cranioplasty; vegetable polyurethane; osteoconduction; osteopromotion
RESUMO
Foi adotado um modelo experimental para avaliar o emprego de um novo biomaterial - uma resina poliuretana vegetal, extraída do óleo de mamona - na reparação de perdas ósseas da calota craniana. Em 31 coelhos adultos, foram criadas duas falhas ósseas cranianas de 15x10 mm de diâmetro, de espessura total e livres de periósteo, em ambos os lados na região parietal. As falhas foram deixadas sem reparação em 13 animais, correspondendo ao grupo controle, e 18 animais tiveram os defeitos reparados com implante de resina poliuretana vegetal. Avaliações macroscópicas, radiológicas e histológicas foram realizadas nos pós-operatórios de 2,6,12,18 e 24 semanas. O grupo controle, por todos os métodos de avaliação, não mostrou reparação dos defeitos por neoformação óssea, com as falhas ósseas ocupadas por tecido cicatricial. No grupo implantado, a partir de 6 semanas, observou-se reparação dos defeitos por neo-osso em suas porções periféricas, evoluindo progressivamente de maneira favorável nas amostras tardias (18 e 24 semanas), podendo-se observar a ocorrência de osteogênese, osteocondução e osteopromoção. As análises estatísticas confirmam que a cicatrização óssea nos defeitos implantados foi mais efetiva de forma significante (p< 0,05). Não foram observados fenômenos tóxicos ou reacionais secundários à presença dos implantes, que não sofreram incorporação no período observado. Não foram evidenciadas propriedades osteoindutivas nos implantes.
Palavras-chave: Cranioplastia; poliuretana vegetal; osteocondução; osteopromoção
A constant search for a tissue substitute of non-vivo material, that originated aloplastics or biomaterials for example, has been occurring for more than a century(1), with different materials being tried. All the problems observed in using bone grafts, particularly for large deformities have encouraged this pursuit(2-4).
Various synthetic resins have been developed and utilized as biomaterials, such as polyethylene(5), ceramics (6-8), silicone(9), titanium (10) and polyrnethylmetacrylate(11-12), thus revealing the complexity of the issue and showing a certain degree of disappointment with the methods of treatment available, and demanding the improvement and search for new materials.
The crucial problem remains to find a material capable of limiting infection, that allows bone growth or bone neogenesis, that has structural resistance and allows reconstruction surrounding sinusal cavities in the craniofacial segment. Location, previous infections and timing of reconstructions are reported as more essential than the choice of the material itself for successful aloplastic repairs in cranioplasties(13).
Adequate assessment of implants for bone repair, and above all of the events involved in the implant-bone relation, is more demanding than simply acknowledging the presence or absence of the conditions that comprise the "biocompatibility" concept. The natural model for analyzing this relationship corresponds to the events observed for using free auto-grafts, with attention given to the phenomena of osteoconduction, osteoinduction, osteogenesis and incorporations, that should be correctly defined and assessed in the presence of implants(14,15,16).In addition to these phenomena, the utilization of implants has added the concept of oriented bone regeneration or "osteopromotion"(l7,18,19), based fundamentally on the difference in repair speed between bone tissue and scar tissue. Since the fibroplasia process(20) is much faster than bone healing, scar tissue eventually replaces the defective area, preventing adequate substitution by the new bone.
VEGETABLE POLYURETHANE RESINS
The Group of Polymer Chemistry and Technology of Universidade de São Paulo - São Carlos has been developing projects for researching and developing vegetable polyurethane resins since 1984, having produced a series of alterna tive polyol and pre-polymer products, synthesized from vegetable fatty acid-derived molecules. The castor oil plant - Ricinus communis, Dicotyledoneae class, Geraniales order, Euphorbiaceae family - is a unique source of these compounds for ricinoleic (12-hydroxioleic) acid, highly pure from a chemical standpoint. The oil extracted is comprised of 81 to 96% of triglycerides from ricinoleic acid, and is considered a natural polyol(21-23).
Polyurethane is obtained through the reaction of a diisocyanate with a polyol. As the compounds contain highly reactive isocyanate groups, a pre-polymerizing reaction with polyol is carried out, increasing its molecular weight- yielding pre-polymers - and leaving a percentage of free isocyanate for the final reaction. Although different classes of isocyanates may be used for the reactions, the pre-polymer adopted was synthesized from diisocianate difenylmethane (MDI) with the polyol derived from castor oil(24). Thus, polymerization of the vegetal PU resin from the castor plant is performed through reactions involving "bicomponent" systems, one of which is the pre-reactive species or pre-polymer (diisocianate n-terminal-MDI + poliol) and the other, the polyol. The proportion between components is 0.65 poliol to 1.00 pre-polymer and adding calcium carbonate corresponding to 50% of the sum of the weight of both components (polyol + pre-polymer).
The system is more attractive for obtaining resins for the following reasons: 1. there is no need for catalysts; 2. simple processing; 3. flexible formulation, allowing for addition of other components without interfering in the reaction; 4. versatility of the "cure" temperature with a maximum exothermic peak of 45°C; 5. no emission of toxic vapors; 6. no residual post-reaction free monomers(23). The vegetable PU resin used in the present study is being analyzed clinically and experimentally for utilization in dentistry, orthopedics, skull reconstructions(25-33)and, in its vulcanized state, as a silicone substitute for testicular prostheses(34). Results have begun to be published, showing its biocompatibility and possibilities of utilization.
In the present experimental model, we aimed to assess the behavior of the polyurethane castor plant resin implant for repairing skull bone defects, analyzing basic osteoconduction, osteoinduction, osteogenesis, incorporation and osteopromotion phenomena.
METHODS
The present study obtained prior approval by the Ethics Committee for Animal Research of Faculdade Estadual de Medicina de São José do Rio Preto.
IMPLANT PREPARATION
We used sterile kits supplied by Instituto de Química Analítica and Tecnologia de Polímeros da USP - São Carlos, with 1 flask with pre-polymer, 1 flask with polyol and a plastic pack with calcium carbonate, and obeyed the following sequence:
• Deposit calcium carbonate in a sterile glass flask.
• Add the flask of pre-polymer, mixing with a sterile spatula.
• Add 1 flask of polyol derived from castor oil and homogenize the mixture for approximately 5 to 8 minutes.
• Handle and model the implants 30 minutes after the polymerization reaction, for the resin to present an exothermal reaction and expand its volume during this period.
SURGICAL PROCEDURES
Thirty six New Zealand rabbits, between 12 and 16 month of age were operated on. There was initial polymer preparation according to the instructions above in order for the animals to receive the implant, taking into consideration that the average polymerization time for the resin was similar to the time necessary for preparing bone failures. Animals were anesthetized with an IM injection of sodium Nembutal, 25 mg/kg, supplemented if necessary until the end of the procedure. The animals were kept prone, and their heads were shaved and sterilized with clorhexedine. After exposing the calvarium by means of a medial cranial incision, we used methylene blue to limit two symmetrical areas approximately 15 x 10 mm in diameter on both sides of the parietal bone region. We made total width periosteum-free bone failures on the areas marked, by manual osteotomy, utilizing a 2 mm scorper. The implants were then modeled and adjusted in terms of defects. The same technical standards were followed for control animals, except that bone failures were kept open without any filling (Fig. 1). Incisions were sutured by planes with Vicryl 4-0. No fixation, local compression or immobilization in the post-operative period was used. Only Rifocin spray was applied localiy for 72 hours. No oral antibiotic or anti-inflammatory treatment was used.
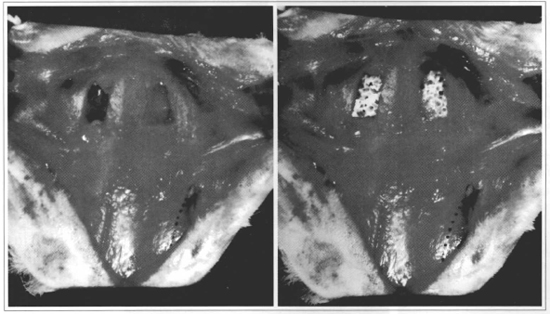
Fig. 1 - Rabbit cranial vault, presenting final aspect of osteotomies. Left - Total width periosteum-free bone failures, exposing dura-mater in their internal surface (final aspect for controlanimals). Right - Bone failures repaired with vegetable PU resin implant (final aspect for implanted animals). Dorted line - Left orbital rim.
SAMPLE ANALYSIS
The animals analyzed were divided into 2 groups as follows: 13 control animals, in which the skull bone failures produced, were not filled; 18 implanted animals, whose bone failures were filled with castor oil plant polyurethane. The batches of control and implanted animals were sacrificed at 2, 6, 12, 18 and 24 weeks, and submitted to macroscopic, radiological, and histological srudies. On the dates planned, the animals of each group were sacrificed with a lethal dose of anesthetic, and the cranial vault exposed, with the removal of its cover in the subgalic plane. The whole calvariurn was removed according to its anatomical boundaries, moment in which macroscopic assessment took place, by sectioning the narurally existing mediocephalic suture. Two specimens were obtained for analysis, totaling 62 samples.
Radiological assessments were carried out for ali samples, using a mammography technique in a high resolution Philips Mammo - UC mammograph, and documented in Kodak Min-R film, sensitized by exposure to 22 KV and 33 MAS. For histological analysis, the material was fixed in 10% formalin for 48 hours, and then submitted to decalcification in a solution of equal parts of 50% formic acid + 20% sodium citrate for an immersion period varying between 18 and 22 days. The slides of the serial 6 µm slices were stained in hematoxilin-eosin and Masson thrichrome, alternatively, and identified as peripheral and central slices.
STATISTICAL METHOD
Radiograms were assessed (Department of Radiology - Beneficência Portuguesa de São José do Rio Preto), determining a scale of values according to the percentage of micro-calcifications present in the defective area (reduction in radiotransparency or size of the defect). The periods that corresponded to the control and implanted groups were analyzed through an unpaired t test with a Welch's correction, and all groups were submitted jointly to Kruskal- Wallis ANOVA, with two tailed tests and a p-value less than 0.05 for level of significance. The analysis was carried out using a GraphPad Instat 3.00 for Windows.
RESULTS
In general, animals tolerated the surgical procedure well. There were 4 deaths, 3 of which occurred in the first 6 post-operative days, secondary to cerebral edema and/or an inadvertent lesion of the dura-mater. Two of the animals were from the control group and one was from the implanted group. The other death was in an animal with 15 weeks of follow-up, due to acute diarrhea, and was an implanted animal.
Regardless of the method of evaluation, the samples from the control group did not present bone neogenesis, and defects were progressively filled by scar tissue. The following observations were made in the implanted group:
MACROSCOPIC ASSESSMENT
At 2 weeks, resin could be observed in the implant site, without adherence to surrounding tissue and with adequate bone fixation. In the remaining animals of the other post-operative periods, visualization of the resin was only possible through the internal surface of the skull, and the external portion presented very firm tissue coverage after skin removal, and the tissue was preserved so as to not affect sample analysis. The internal portions had a progressive reduction of the defect, with evident loss of its initial geometric format, and portions of resin covered or interwoven with probable bone tissue. No abnormal secretions or collections were observed in the operated areas or surrounding tissues.
RADIOLOGICAL ASSESSMENT
At 2 weeks resin radiotransparency became evident, without any point of microcalcification. At 6 weeks, there were irregular areas of microcalcification, occupying the peripheral portions of the defective region. The process expanded progressively in time, radiotransparency decreased, and a regular calcification gradually occupying the defective space was observed (Fig. 2). All radiograms showed the margins of the bone failure produced as a "scar" of the fracture performed. Both samples did not show microcalcifications in the implanted region only in 1 animal in the 24 week group.
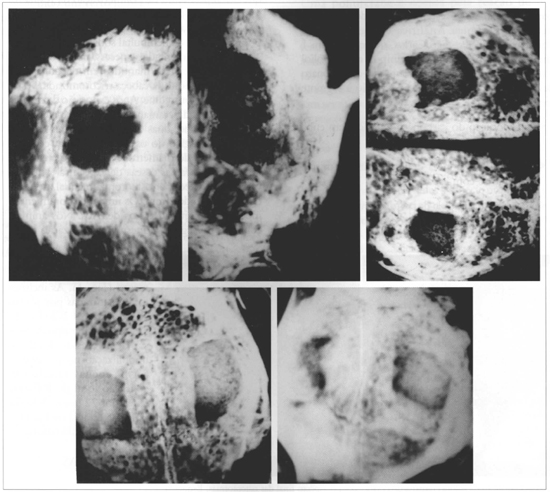
Fig. 2 - Radiograms of the cranial vault of samples of the implanted group, in the different post-operative periods. Upper Left-6 weeks; Upper Center - 12 weeks; Upper Right - 18 wceks; Lower left and right - 24 weeds. Progressive reduction of radiotransparency Micro-calcifications visibly initially on peripheral portions, gradually filling defects (Mammograph Mammo UC -Kodak Min-R - 22 KV/33MAS Film).
HISTOLOGICAL ASSESSMENT
At 2 weeks, adequate implant filling of the defect could be observed, along with slight osteogenic activity on the margins of the fracture and a moderate inflammatory infiltrate. At 6 weeks, a continuous cortical bone formation could be seen, covering the defect from its margins, concentrically, with non-closing central areas. The neoformation always occurred on the internal surface of the defect. At the external periosteum-free portion only resin covered by a thin layer of fibrous tissue was present (Fig. 3). The beginning of a slight irregular fragmentation of the resin could be seen at the implant - bone transition. At 12 weeks, bone cortical was present only on the endocranial face. It had a denser aspect with more intense micro-fragmentation of the implant and initial process of "transresin" bone neoformation (Fig. 4). The findings in the 18 and 24 week samples were an extension of these observations, with thicker transresin bone trabeculae, ossification on the internal surface of the defect, absence of foreign body type reaction or formation of a capsule around the implant (Fig.5). There was no bone growth in both sampies on one animal and the implant was practically intact, without fragmentation.
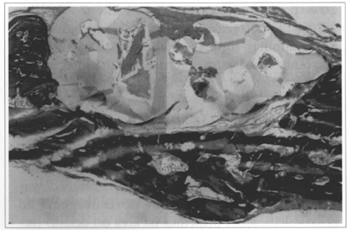
Fig. 3 - Sample of an implanted animal at 6 weeks. Implant filled the bone failure completely, with bone neoformation visible on the endocranial portion. External portion showing a thin layer of fibrous tissue (Col. TM, 40x enlargement).
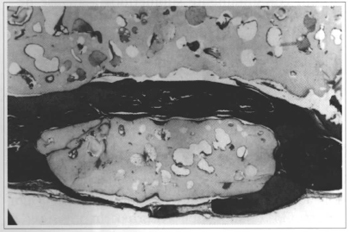
Fig. 4 - Sample of an implanted animal at 12 weeks. "Transresin" bone neoformation by implant fragmentation (Col. TM, 60x enlargement).
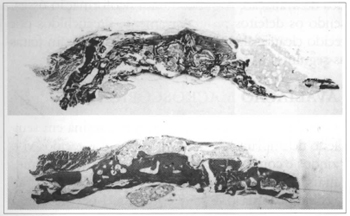
Fig. 5 - Upper - Sample of an implanted animal at 18 weeks. Fragmentation and dislocation of the implant by new-bone, with varied-size fragments (Col. TM, 5x enlargement). Lower - Sample of an implanted animal at 24 weeks. Dense and thick aspect ossification on the endocranial Portion. No inflammatory of foreign body reaction (Col. EO 5x enlargement).
STATISTICAL ANALYSIS
The number of microcalcifications in the implanted defects was significantly higher (p< 0.05) than in controls for the correspanding periods. Simultaneaus analysis of all samples showed that bane healing in the failures repaired was very significantly more etfective (p< 0.001).
DISCUSSION
The experimental model adopted tried to reproduce the usual situatians seen in clinical practice, particularly in immediate reconstructians, with proportionally large bane failures, affecting the whale bane width, and totally free of periasteum, making the implanted material very important for the healing process.
Although the experiment could have been carried out with the implant and control in the same animal, we chose to do it separately, so the general behavior of the animals receiving implants could be observed. In this sense, the 3 deaths in the immediate post-operative period, representing 10% of animals operated on, were considered as a direct consequence of the surgical aggression and not secondary to any toxicity of the implants, considering that two of them occurred in control animals.
The in situ preparation of implants allowed the assessment of their handling and plasticity characteristics that were satisfactory, withour technical preparation and molding difficulties. However, during the implant polymerization process there was a major volume expansion, persisting to roughly 30 to 40 minutes of reaction. The choice of the mammography technique for radiological assessment was due to its acknowledged sensitivity for detecting micro-calcifications(35).
In general, the observations showed the biocompatibility of the implant used. No local or systemic reactions - late ar immediate-, extrusion, capsule formation around the implant ar foreign body reactions were observed, confirming findings of other studies(24,27,28,30).
The presence of post-reaction free residual monomers in polymerization reactions is directly related to the toxicity of these polymers when they are used as implants(36,37). The bicomponent (poliol + pre-polymer) reaction used for synthesizing this kind of implant does not allow for "leftover" radicals, due to its non- monomeric origin, and ir may be one less factor in the development of toxic or reactional phenomena. Also in regard to in situ polymerization processes, the cure temperature may be an essential factor in tissue damage(38,39).The maximum exothermal peak reached in the beginning on the liquid-gel transition of the castor polymer mixture is around 45°C(24). In addition to being a relatively low temperature during this phase, that occurs between 8 and 15 minutes after the mixture, in the present study, the resin was still not in contact with the donor area and was implanted 30 to 40 minutes after polymerization, which was already undergoing major cooling. No thermal damage to tissue was observed, particularly to the brain tissue in intimate contact with the implant.
The concern with the mechanical resistance of bane substitute implants seems obvious, particularly for long bones(40,41).In cranioplasties, and implants of the orbital floor, malar region and nasal dorsum, the absence of movement and the low incidence of mechanical forces are described as facilitating factors for utilizing implants, making these regions more favorable for its utilization(42). We were not able to quantify if the fragmentation observed affected mechanical resistance of the implanted site, because concomitant bane growth was observed, which is essential information for using the implant in areas with greater mechanical stress.
The importance of implant porosity in osteoconduction phenomena, is being stressed since the introduction of ceramics and polyethylenes as bane substitute implants(45,46), although still a controversial matter, due to the wide structural diversity presented, regarding the differences in the diameter of pores and their ideal size (47,48,49). PU castor resin has a "virtual" pore structure resulting from its expansion during polymerization, and is not a true canalicular system of intercommunicating pores. This did not prevent osteoconduction, as transresin bone growth occurred as a result of fragmentation, except in 1 animal. The fact probably shows the difference in the physical features of the implant, during preparation or polymerization time, suggesting that the degree of compactation may interfere directly in the osteoconduction of recent fractures.
The bone neoformation that is always seen at the internal face of the defect (endocranial) and transresin are osteopromotion (43,44), in which the resin is having a "membrane effect" in the implanted areas, preventing it to be replaced by fibroplasia, as was the case in controls. The dura-mater, in the internal surface of the defect, may have played a major role in supplying osteogenic elements to the healing process, in addition to having a membrane effect on the endocranial portion. The absence of new bane on the external portion of the failure confirms the importance of the periosteum as an osteogenic element, in addition to demonstrating that the periosteum also plays a role as a "natural membrane", enhancing the importance of its integrity during treatment. Some studies have demonstrated that clinical effectiveness of repair by means of osteoinduced growth requires some kind of material filling the defect, both to exert the membrane effect, and also to support and be a potential means of transportation(50,51).
The assessment of the osteoinductive properties in implants is a difficult task because induction mechanisms are not totally known and there may be some confusion with bone growth stimulation or modulation. In the present study, the reconstruction was carried out immediately, thus favoring healing processes. Slow osteogenesis and osteoconduction in the defects repaired demanded neoangiogeneis and neovascularization, bringing along undifferentiated mesenchymal cells, with a differentiation potential to form bone tissue. Studies have demonstrated that intramembranous (IM) bone grafts induce bone neoformation through IM ossification, while cortical bones undergo endocondral (EC) ossification (52,53). Although almost all known osteoinductive factors have been isolated from EC bones, recent studies have demonstrated that these factors may be present in the bone tissue that is completely formed through IM ossification (54). In this manner, induction for mesenchymal cell differentiation probably originated from the margins of the receptor site, from a local inducer, and the implant represents only an osteomodulating substrate, without osteoinductive properties.
From the histological standpoint, no phagocytic activity with cell reabsorption of implants was observed. Despite bone growth, no volumetric increase was observed in the implanted areas, which is not apparently justified only by the fragmentation seen in the implants. Some authors, that have made the same observation, suggest the possibility of some form of partial replacement of the resin or a "metabolization" process, with fatty acid chains undergoing a similar process of lipid decomposition on the implant surface, along with weakening of the hard segments of the polymer(28,30). At any rate, complete incorporation of implants was not observed until 24 weeks of follow-up.
Castor polyurethane resin is being added to the therapeutic armamentarium of bone healing. The unprecedented biological phenomena observed in the implant-bone relationship requires deepening clinical and experimental research, particularly in respect to possible resin metabolization processes and to osteoconduction and osteomodulation phenomena.
CONCLUSIONS
The castor polyurethane resin implant proved to be biocompatible and to present characteristics that allow its addition to the therapeutic armamentarium for cranioplasties. Osteogenesis, osteoconduction and osteoprornotion were observed in bane failures, alang with progressive and satisfactary bane neoformation as of 6 weeks. Implants did not underga complete incorporation or present evidence of osteoinductive proprieties until 24 weeks of follow-up.
REFERENCES
1. Booth JA, Curtis BE Report of a case of tumor of the left frontal bone. Ann Surg. 1899; 17:127-9.
2. Craft PD, Mani MM, Pazel J. Experimental study of healing in fractures of membranous bone. Plast Reconstr Surg. 1974; 53:321-8.
3. Enneking WF, Eady JL, Burchardt H. Autogenous cortical bone grafts on the construction of segmental defects. J Bone Joint Surg. 1980; 62 A:1039-45.
4. Munro IR. Split rib cranioplasty. Ann Plast Surg. 1981; 7:341-4.
5. Berghaus A. Porous Polyethylene in Reconstructive Head and Neck Surgery. Arch Otolaryngol. 1985; 111: 154-60.
6. Salyer KE, Hall CD. Porous hidroxiapatite as an onlay bone graft substitute for maxillofacial surgery. Plast Reconstr Surg. 1989; 84(2) :236-44.
7. Jarcho M. Calcium Phosphate Ceramics as Hard Tissue Prosthetics. Clinical Orthop and Rel Research. 1981; 157:259-78.
8. Miller TA, Ishida KB, Kobayashi M, Mollman JS, Turk AE, Ralph HE. The induction of bone by an osteogenic protein and the conduction of bone by porous hydroxyapatite: A laboratory study in rabbit. Plast Reconstr Surg. 1991; 87(1):87-95.
9. Chicarilli ZN, Ariyan S. Cranioplasty with a silicone prosthesis and split rib grafts. Head Neck Surg. 1986; 8:355-62.
10. Joffe JM, Aghabeiji B, Davies EH. A retrospective study of 66 titanium cranioplasties. British J Oral Maxillofac Surg. 1993; 31:144-8.
11. Van Gool AV. Preformed Polymethylmetacrylate Cranioplasties. J Maxillofac Surg. 1985; 13(1):2-8.
12. Remsem K, Lawson W, Biller HE Acrylic frontal cranioplasry Head Neck Surg. 1986; 9:32-6.
13. Manson PN, Crawley WA, Hoopes JE. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986; 7(6):888-900.
14. Burchardt H. The biology of bone graft repair.Clin Orthop Rel Res. 1983; 74:28-41.
15. Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971; 50(6): 1392-7.
16. Reddi AH, Wientroub S, Muthukumaran N. Biologic principies of bone induction. Orthop Clin North Am. 1987; 18:207-12.
17. Dahlin C, Linde A, Gottlow J. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988; 81(5):672-6.
18. Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984; 11:494-9.
19. Linde A, Alberius P, Dahlin C. Osteopromotion: A soft tissue exclusion principie using a membrane for bone healing and bone neogenesis: A review article. J Periodontol. 1993; 64:1116-29.
20. Ackerman AB. Histologic diagnosis of inflammatory skin diseases. 2. ed. Baltimore: Williams & Wilkins; 1997. p. 105.
21. Bayer O. Angew Chem. 1947; 59:247 Bios Report 628, item 22.
22. Araújo LC. Caracterização química, térmica e mecânica de poliuretanas elastoméricas baseadas em materiais oleoquímicos [Tese de Mestrado]. IFQSC/USP; 1992.
23.Claro Neto S. Caracterização físico-química de um poliuretano derivado do óleo de mamona utilizado para implantes ósseos [Tese de Doutorado]. Instituto de Química de São Carlos - USP; 1997.
23. Carvalho TLL, Araújo CAC, Teófilo JM, Brentegani LG. Histologic and histometric evaluation of rat alveolar wound healing around polyurethane resin implants. Int J Oral Maxillofac Surg. 1997; 26:149-52.
24. Cavalca D. Uso do composto ósteo-ricinus em reconstrução de perdas ósseas em ortopedia. Anais I Jornada de Ciência e Tecnologia de Biomateriais; 1998 Set 4-5; Vitória-ES, Brasil; 1998.
25. Caruzzo SL. Implantes de resina poliuretana vegetal em arco zigomático de ratos - Estudo histológico. Anais IX Jornada Acadêmica de Araraquara. Jornada Acadêmica de Araraquara; 1995 Nov 14-15; UNESP
26. Ohara G, Kojima E. Estudo experimental da biocompatibilidade do polímero poliuretano da mamona implantado intra-ósseo e intra-articular. Acta Ortop Bras. 1995; 3(2).
27. Kharmandayan P. Estudo da interface de contato entre osso e implantes de poliuretano com e sem carbonato de cálcio, empregando microscopia eletrônica de varredura em coelhos [Tese de Doutorado]. Univ Federal de São Paulo - Escola Paulista de Medicina; 1997.
28. Oliveira MF, Ueda JK, Rezende D. Estudo comparativo entre a reparação com biomaterial poliuretano derivado do óleo de mamona e osso autógeno em calvária de coelhos - Análise Histológica. Anais IX Jornada Acadêmica de Araraquara; 1995 Nov 14-15; Universidade Estadual Paulista - UNESP
29. Ignácio H. Utilização do cimento derivado do polímero da mamona no preenchimento de falha óssea. Estudo experimental em coelhos [Tese de mestrado]. Departamento de Ortopedia e Traumatologia - Faculdade de Medicina de Ribeirão Preto - Universidade de São Paulo; 1995.
30. Ara CS. Experiência de 3 anos com o uso do polímero de Ricinus comunnis em intervenções neurocirúrgicas. Anais I Jornada de Ciência e Tecnologia de Biomateriais; 1998 Ser 4-5; Vitória- ES; Brasil; 1998.
31. Ramalho LTO. Biocompatibilidade da resina poliuretana vegetal derivada do óleo de mamona: Estudos histológicos. Anais I Jornada de Ciência e Tecnologia de Biomateriais; 1998 Set 4-5; Vitória- ES; Brasil; 1998.
32. Frascino LF. Implante de resina poliuretana vegetal no segmento craniofacial. Anais I Jornada de Ciência e Tecnologia de Biomateriais; 1998 Set 4-5; Vitória-ES; Brasil; 1998.
33. Costa RP, Shall CH. The use of "Ricinus comunnis" polymer as a silicone substitute: a new material for prosthesis. Recent Advances in Plastic Surgery, São Paulo, March 14-15, 1992.
34. Rocha CD, Bauab SP Atlas de Imagem da Mama. Correlação entre mamografia e ultra-sonografia. 1. ed. São Paulo: Sarvier; 1995. p. 15-1995.
35. Fletcher AM, Purnaveja S, Arnin WM, Wood AW. The level of residual monomer in self-curing denture base materials. J Dent Res. 1983; 62:118-20.
36. Kaaber S, Thulin H, Nielsen E. Skin sensivity to denture base materiais in burning mouth syndrome. Contact Dermatitis. 1979; 5:90-6.
37. Hammon WM, Kernpe LG. Methyl Metacrilate Cranioplasty - 13 years experience with 417 patients. Acta Neurochir. 1971; 25:69-77.
38. Asimacopoulos TJ, Papadokis N, Mark VH. A new method of cranioplasty. J N eurosurg. 1977; 47:790-2.
39. De Conti OJ. Estudo experimental da utilização de cerâmica no preenchimento de falha óssea [Tese de Mestrado J. Faculdade de Medicina de Ribeirão Preto - USP; 1997.
40. Schimitt H, Fournier JA, Skondia V. The use of a biocompatible orthopedic polymer in the treatment of loose total hip prosthesis. J Int Med Res. 1989; 17(3):254-61.
41. Spiessl B. New Concepts in Maxillofacial Bane Surgery. Springer Verlag; 1976. p. 125.
42. Linde A, Alberius P, Dahlin C. Osteopromotion: A soft tissue exclusion principie using a membrane for bone healing and bane neogenesis: A review article. J Periodontol. 1993; 64: 1116- 29.
43. Lundgren D, Nyman S, Mathisen T. Guided bane regeneration of cranial defects using biodegradable barriers: An experimental pilot study in rabbits. J Maxillofac Surg. 1992; 20:257-69.
44. Bolander ME, Balian G. The use of demineralized bane matrix in the repair of segmental defects. J Bane Joint Surg. 1986; 68(A):1264-74.
45. Cestero HJ Jr, Salyer KE, Toranto IR. Bone growth into porous carbon, polyethylene and polypropylene prostheses. J Biom Mater Res, 1975; 9:1-7.
46. Bucholz RW, Carlton A, Holmes RE. Hydroxyapatite and tricalcium phosphate bane graft substitutes. Orthop Clin North Am. 1987; 18(2):323-34.
47. Koster K, Heide H, Konig R. Resorbable calcium phosphate ceramics under load. Langenb Arch Chir. 1977; 343:173.
48. Jarcho M. Calcium Phosphate Ceramics as Hard Tissue Prosthetics. Clinical Orthop and Rel Research. 1981; 157:259-78.
49. Rabie ABM, Deng YM, Hägg U. The effect of demineralized bone matrix on the healing of intramembranous bone grafts in rabbit skull defects. J Dent Res. 1996; 75(4):1045-51.
50. Habal MB, Reddi H. Bone grafts and bane induction substitutes. Clin Plast Surg. 1994; 21(4):525-42.
51. Mckibbin B. The biology of fracture healing in long bones. J Bone Joint Surgery. 1978; 60(b):150-62.
52. Scott CK, Hightower JA. The matrix of endocondral bones differs frorn the matrix of intramembranous bone. Calcif Tissue Int. 1991; 49:349-54.
53. Scott CK, Bain SD, Hightower JA. Intramembranous bone matrix is osteoinductive. Anat Rec. 1994; 238:23-30.
I - Head of the Plascic Surgery Service - Beneficência Portuguesa de São José do Rio Preto. Post-Graduate Research Division - Faculdade Estadual de Medicina de São José do Rio Preto; Member of the SBCP
II - Head of the Post-Graduation Program - Faculdade Estadual de Medicina de São José do Rio Preto; Head of the Cardiology and Cardiovascular Surgery Service - Faculdade Estadual de Medicina de São José do Rio Preto; Head of the Cardiovascular Surgery Service - Universidade Estadual de Campinas.
Address for correspondence:
Luiz Fernando Frascino, MD
R. Antonio de Godoy, 3945 - Redentora
15015-100 - São José do Rio Preto - SP Brazil
Phone: (55 17) 233-7439
e-mail: luizfrascino@lmimedriopreto.com.br


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter