

Original Article - Year 2017 - Volume 32 -
Establishment of a protocol for storage of refrigerated autologous skin
Estabelecimento de protocolo para armazenamento de pele autógena refrigerada
ABSTRACT
INTRODUCTION: Autologous skin grafts are used for treatment of burn patients. These grafts can be stored and preserved, as long as the storage process is performed with strict quality control to reduce the risk of infection.
METHODS: A retrospective cohort study was conducted in the Burn Unit of the Hospital das Clínicas de São Paulo from February 2015 to July 2016. During this period, a protocol was established to store refrigerated skin, with control of collection, preservation, and packaging, and recording of all processes. To ensure quality, graft biopsies were collected for pre- and post-storage microbiology testing and a cross-sectional study for contamination was performed.
RESULTS: Critical deficiencies included inadequate packaging, lack of processing records, lack of biopsies for microbiology testing, and failure to discard specimens. Most of the samples were contaminated before and after storage (84.2%). Only two samples were sterile before storage but became contaminated after storage, with growth of Gram-positive skin bacteria.
CONCLUSION: A promising method for the storage of refrigerated skin was established, but requires minor adjustments in quality control.
Keywords: Autologous transplantation; Skin grafting; Tissue preservation; Quality control; Refrigeration.
RESUMO
INTRODUÇÃO: Enxertos de pele autólogos são utilizados em tratamento de pacientes queimados. Esses enxertos podem ser armazenados e preservados, desde que o processo de armazenamento seja realizado com rígido controle de qualidade, para garantir a redução dos riscos de infecção.
MÉTODOS: Foi realizado um estudo de coorte retrospectivo na Unidade de Queimados do Hospital das Clínicas de São Paulo no período de fevereiro de 2015 a julho de 2016, em que foi estabelecido um protocolo para armazenamento de pele refrigerada com controle de coleta, preservação, embalagem e registro de todos os processos. Para garantia de qualidade, foram coletadas biópsias dos enxertos para microbiologia pré e pós-armazenamento e realizado um estudo transversal de prevalência de contaminação pré e pós-estocagem.
RESULTADOS: Os pontos críticos encontrados foram inadequação de embalagem, ausência de registros de processos, falta de coleta de biópsias para microbiologia e falhas no descarte. A maior parte das amostras estava contaminada tanto pré como pós-estocagem (84,2%). Apenas dois pacientes apresentaram microbiologia estéril no pré e contaminada no pós, porém foram encontrados germes da pele do tipo gram+.
CONCLUSÃO: Foi estabelecido um método promissor de armazenamento de pele refrigerada que necessita alguns pequenos ajustes para adequação ao controle de qualidade.
Palavras-chave: Transplante autólogo; Transplante de pele; Preservação de tecido; Controle de qualidade; Refrigeração.
The treatment of burn patients is based on the use of autologous skin grafts, in which the surgeon removes skin from the patient's own donor site and covers the wound during the same surgery. However, sometimes the lesion bed is not ready for grafting, or the surgeon removes excess skin that could be used to treat other wounds in future or possible areas of graft integration failure. Thus, secure skin storage and preservation until needed for a new surgical procedure would be useful1.
The storage of refrigerated autologous skin grafts was first evaluated by Wentscher in 1903, using a storage period of 14 days at 8ºC2. Other studies were conducted in 1912 by Carrel, who examined skin preservation at normothermic and hypothermic temperatures, and found that skin could be maintained in hypothermia for up to two weeks3.
Clinical use of refrigerated skin was first reported during the Second World War. Burn wounds underwent multiple surgeries under general anesthesia. To reduce the surgical time, surgeons took several autologous skin grafts at once and kept them refrigerated until the raw areas were prepared to receive the graft4.
During the same period, Mattews described a method for storing refrigerated skin wrapped in netting or gauze and soaked in saline solution to prevent dehydration. These were placed in hermetically sealed vials and stored at 3-6ºC. It was found that the optimal storage time was between 2 and 8 weeks and that some samples showed contamination by normal skin flora4.
After verifying viability of refrigerated skin for up to 2-3 weeks4,5, the current concern is the risk of contamination. Titley et al.5 carried out a qualitative and quantitative bacteriological study on 102 refrigerated autologous skin graft specimens. Culture was performed on harvesting, during use, and after 21 days of storage.
The authors found that storage conditions influenced the growth of microorganisms and that the count per gram of skin (organisms/g) might interfere with graft integration. The association between graft attachment and number of organisms/g at the time of harvesting was relatively weak (r = -24); however, most of these grafts (96.4%) had a contamination rate of <105 organisms/g. After three weeks of storage, about 40% of the grafts showed contamination with >105 organism/g. The authors attributed this finding to storage in a refrigerator with fluctuating and non-standardized temperatures5.
Thus, refrigerated skin storage must be performed with strict quality control to reduce infection risk. The process of harvesting, packing, and storage must follow the precepts of good practice using aseptic technique, properly standardized material, a refrigerator for exclusive use with standardized and monitored temperature, and oversight of all procedures by a multidisciplinary team5,6.
Since the 1950s, the Burn Unit of the Hospital das Clínicas of the University of São Paulo has been storing refrigerated tissue for autologous transplantation. However, this was done with little control or concern for packaging, absence of standardization of the storage processes, missing data records, and without evaluation for contamination7.
With the introduction of hospital accreditation and quality control systems, the nursing team of the Burn Unit and the Tissue Bank, assisted by medical technicians and physicians, felt the need to establish new standardized and controlled protocols for the preservation and storage of refrigerated autologous skin.
OBJECTIVE
The objective of this study was to evaluate a model for the storage of refrigerated autologous skin using the results of microbiological cultures to ensure quality control.
METHODS
This was a retrospective cohort study performed at the Burn Unit of the Hospital das Clínicas de São Paulo from February 2015 to July 2016.
This study followed the principles of the Declaration of Helsinki and was exempt from review by the Research Ethics Committee, since it was a management study focused on improving an existing process, to be used in validating a method for autologous skin storage.
Place of study: Burn Unit and Tissue Bank of the Central Institute of the Hospital das Clínicas de São Paulo.
Phase 1 - Description of the standard model for refrigerated partial-thickness skin storage for autologous use
1A - Skin harvesting phase
Patients with second- and third-degree burns admitted to the Burn Unit of the Hospital das Clínicas underwent skin grafting using standard criteria, without involvement of the research group in assessing treatment indications. Harvesting of skin grafts was performed in a surgical center under general or spinal anesthesia, and was always performed by a plastic surgeon experienced in sterile technique.
The donor sites were disinfected with 2% chlorhexidine for 5 minutes and antisepsis was performed with 0.5% alcoholic chlorhexidine. Partial skin harvesting was performed with an Integra® brand dermatome with thickness ranging from 12-18 µm. When more graft skin was harvested than needed, the plastic surgeon immediately informed the nurse, who started the storage process.
1B - Processing phase
When advised of the need for storage, the nurse provided a packaging kit (preparation described below), Ringer's Lactate (100 ml), and a bottle of sodium thioglycolate for microbiological culture.
Initially, the physician took a small sample for biopsy and placed the tissue in a bottle of sodium thioglycolate using sterile technique for a pre-storage control culture.
The primary bottle with the skin sample was then deposited in sterile packaging and completely covered with Ringer's Lactate solution, then sealed. The first package was then inserted into a second container in such a way that the first package was no longer accessible. The nurse then sealed the second package with a Ron Micromecânica RSR - 2000 model sealer.
A label with patient identification, refrigerated tissue type, processing date, and expiration date was placed on the package, which was resealed. Thus, the final package had a double seal with proper identification and was ready for storage.
1C - Storage phase
After packing, the graft was stored in a Consul Compacto 120 refrigerator at 4ºC±2ºC, located in the surgical center of the Burn Unit. The skin was kept in a refrigerator until use or for a maximum of 14 days. At the time of use or disposal, a new tissue biopsy sample was collected for post-storage microbiological analysis.
1D - Packaging preparation
An important step in the establishment of the protocol was carried out when the packaging was designated for skin conditioning. For this choice, the norms defined in the current resolution were followed. These defined the necessary packaging specifications as well as their physicochemical, cytotoxic, and pyrogenic properties8. Prior to use, the primary and secondary packages were sterilized in ethylene oxide (Figure 1).

Figure 1. Packaging for storage of autologous skin grafts.
Maxvac® was used for autologous skin graft storage, and was certified for the following: heat sealing, puncture resistance, tensile strength, oxygen and water vapor permeability, and migration. Cytotoxicity and pyrogenicity tests were carried out in the laboratories of the Adolfo Lutz Institute and Medlab, respectively. The packaging was pyrogen-free with no toxic effects on cell lines. Thus, it was possible to define the quality of tissue storage packaging.
1E - Records of procedures
Ensuring quality control depends on proper recording of all procedures. In this stage, instruments were created to record patient information with the following data: name, sex, registration number, date of birth, tissue withdrawal, biopsy specimen sent to the microbiology laboratory, and the use and disposal of the grafts.
To ensure traceability, a counter-referral system was created in which the patient's name, registration, and stored tissue type were identified on the label attached to the packaging, on the Preoperative Nursing Assistance Protocol form located on the medical record, and in the general control book for refrigerated autologous skin grafts.
The storage refrigerator was monitored with a digital Incoterm® maximum-minimum thermometer and checked three times a day, with data recorded on a Temperature Control Record Sheet. The refrigerator was cleaned every two weeks with water and neutral detergent, and the stored material was held in another special test sample cooler for 2 to 3 hours until the temperature stabilized in the clean refrigerator. During this temporary storage, the tissue was kept in double-sealed packaging, with no samples for analysis in the refrigerator.
Phase 2 - Microbiological testing to evaluate the efficacy of the refrigerated autologous skin storage protocol
This phase performed microbiological testing to evaluate contamination of the stored material. From February 2015 to July 2016, data were collected on patients who had skin grafts stored by refrigeration at the Burn Unit.
The information obtained was recorded in a collection instrument with the following data: name, registration number, date of birth, sex, date of harvesting, transplantation, or disposal, and pre-storage and pre-transplant or post-storage culture results (14 days of preservation). Microbiological analyses were carried out for aerobic and anaerobic bacteria and fungi and the test results were verified through a computerized testing system.
2A - Inclusion and exclusion criteria for microbiological testing
The inclusion criteria for sample testing were: autologous grafts stored with all fields completed on a registration sheet used for control of autologous skin grafts in burn victims, with microbiological evaluation performed for both pre- and post-storage skin biopsies; and grafts with pre- and post-storage microbiological analyses (for anaerobic and aerobic bacteria and fungi), with conclusive final results, i.e., without bacterial growth or identification of a contaminating microorganism.
Exclusion criteria: autologous grafts in which the biopsy was only performed pre- or post-storage; or culture results that were consistently inconclusive.
2B - Analyses of the results
From the data collected, simple statistics could be used to determine the characteristics of the sample, the transplantation and material disposal rates, and the contamination rates before and after storage, for identification of any contamination during the storage process.
RESULTS
Of 88 autologous skin graft specimens collected, 50 (57%) were discarded, 27 (54%) were biopsies collected for post-storage testing, 17 (34%) were collected for pre-storage testing, and 6 (12%), biopsies were not collected for testing.
Of 38 (43%) samples studied, 34 (89.5%) were contaminated; of these, 27 (71.1%) had pre-storage contamination that persisted in post-storage culture, 5 (13.1%) were only contaminated pre-storage, and 2 (5.3%) were only contaminated post-storage. The average storage period was 14 days.
No contamination was detected in pre- and post-storage testing of 4 (10.5%) samples. However, 32 (84.2%) showed contamination in pre- and post-storage testing. The main microorganisms found were: Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, Klebsiella pneumoniae, and coagulase-negative Staphylococcus.
The main difficulties encountered in standardization of the storage method were:
a) sealing of secondary packaging - the lack of a portable sealer made it difficult to move the equipment to surgical centers other than the burn unit;
b) information recording - the lack of initial designation of a nurse responsible for control of stored autologous skin grafts was due to lack of records, incomplete records, and difficulty tracing information;
c) samples stored longer than 14 days - the lack of initial designation of a nurse responsible for control of the autologous skin graft storage cooler led to storage and disposal of samples beyond 14 days;
d) microbiological analysis - the lack of biopsy collection in the pre- and/or post-storage period by the medical team resulted in missing or incomplete microbiological results.
Figure 2 shows the main sources of deficiencies in biopsy collection for microbiological examination.
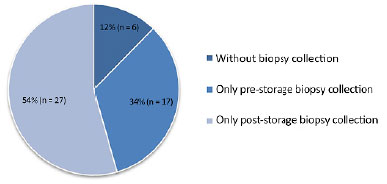
Figure 2. Causes of graft exclusion from the study.
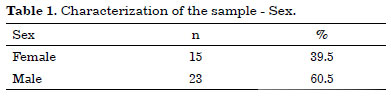
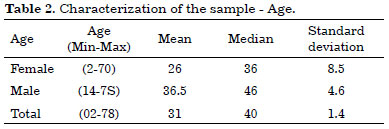
Thus, the infection control study of 38 refrigerated skin grafts represented 43% of the total number of patients with stored tissue. Most patients were males with a mean age of 36.5 years (Tables 1 and 2).
The prevalence of contamination was associated with four possible situations for each graft: contamination in pre- and post-storage culture, contamination only in pre-storage culture, contamination only in post-storage culture, and absence of contamination in either the pre- or post-storage culture. Figure 3 shows this distribution.
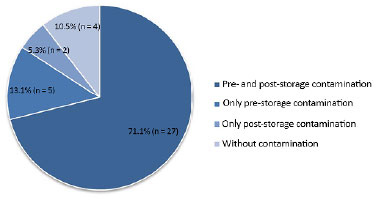
Figure 3. Prevalence of contamination in stored autologous skin grafts.
In situations with contamination in both pre- and post-storage culture, in pre-storage culture alone, or in culture without contamination, we were able to confirm that storage conditions were not the source of bacteria; however, contamination in the post-storage culture alone (2 patients, 5.3%) caused greater concern, and required more detailed analysis of the type of organisms found (Table 3).
Figures 4 and 5 list the main microorganisms found in pre- and post-storage cultures, respectively.
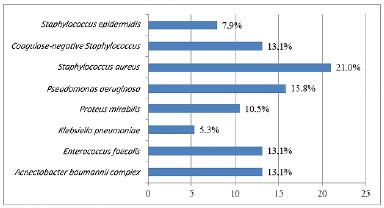
Figure 4. Bacteria isolated in the pre-storage culture of 38 autologous skin grafts.
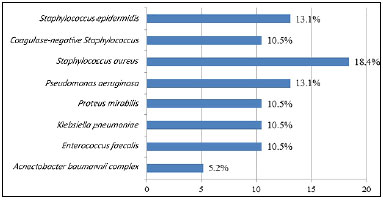
Figure 5. Bacteria isolated in the post-storage culture of 38 autologous skin grafts.
Regarding final tissue destination, only 3 of the 38 grafts were used for transplantation (7.9% of the sample), with a mean storage time of 6.5 days. All other material was discarded.
DISCUSSION
The development of a standardized method for the storage of refrigerated autologous skin is key to ensuring quality control for the entire process of harvesting, storage, use, and disposal of autologous grafts. The main objective of this study was to establish a protocol that would involve the entire multidisciplinary team in the burn unit, with a fundamental role for the nurses who would ensure the execution of the process with maximum quality control.
During establishment of the protocol, it was essential to identify critical points that could injure the tissues and recipients. The main critical points were related to packaging of the material, recording of processes, disposal of materials, and collection of microbiological samples.
The evaluation of the packaging required complementary studies for mechanical concerns and toxicity of materials; however, it was difficult to make the team aware of the need to use previously standardized materials. Some of the staff stored the tissue in improper packaging and insisted that this needed to be kept in the skin storage refrigerator. However, this attitude could not be tolerated, because the risk of cross-contamination within the refrigerator greatly increased with storage of packages without appropriate certification12.
Other critical points (failure in recording, discarding of material, and collection of cultures) were identified during the microbiological contamination prevalence study. The processes were not adequately annotated, many tissues were out of storage and were not discarded, and some microbiological cultures were not collected during pre- and post-storage.
To solve these problems, a nurse was designated as the professional responsible for the control of all activities. This made it possible to ensure improvement in the collection and storage of information, and that the entire process was performed safely.
Concern about the risk of contamination in the standardization of our model was so important that it required a complementary study on contamination prevalence in the stored material. The final sample of the study consisted of 38 refrigerated grafts, with a predominance of male patients (60.5% × 39.5%) and a mean age of 31 years, indicating the predominance of economically active individuals.
In our results, only 4 (10.5%) of grafts had no pre- or post-storage contamination. On the other hand, 32 (84.2%, pre- and/or pre- and post-storage contamination) were contaminated prior to refrigeration. Thus, we can infer that antisepsis and sterilization of donor tissues were inadequate or that patients had excessive skin contamination, with inability to adequately reduce the bacterial load at the time of harvesting.
The main microorganisms found were S. aureus, P. aeruginosa, E. faecalis, K. pneumoniae. and coagulase-negative Staphylococcus, these being the most common agents in large burns. Li et al.13 found a similar microbial profile in 30 skin graft samples kept in refrigeration, with polymicrobial growth in 43% of the cultures.
However, the most relevant finding was that samples that were not contaminated in pre-storage testing became contaminated after storage (2 samples, 5.3%). Therefore, these cases were analyzed individually, to determine whether the standardized methodology was feasible. In both situations, the microorganisms found were Gram-positive bacteria, which are usually present in the skin normal flora.
Titley et al.5 also studied refrigerated storage of skin by conducting qualitative and quantitative tests for contaminating bacteria, and correlated the findings to the graft integration rate. They demonstrated that skin contaminated with only Gram-positive bacteria, even with more than 105 colonies, had high rates of integration with no infection in the recipient, whereas those with Gram-negative growth had complete loss of transplanted grafts. They also demonstrated that contamination of previously sterile grafts probably occurred because of the use of home refrigerators for storage.
These devices undergo large temperature fluctuations and can provide conditions for bacterial growth. This was probably the reason for 2 cases of bacterial growth in post-storage culture. The home refrigerator used in our burn unit was not able to maintain a stable temperature. Thus, we requested acquisition of scientific refrigeration equipment.
The main limitation of this study was the lack of quantitative cultures to better identify the existing bacterial load in stored material. New studies are already being planned to improve the storage model for tissue implanted in our service.
CONCLUSION
Given the results, it was possible to develop a promising method for storage of autologous refrigerated skin, but further study and standardization are required for adequate quality control.
COLLABORATIONS
ROC Analysis and/or interpretation of data; statistical analyses; final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
AOP Analysis and/or interpretation of data; statistical analyses; final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
EFP Final approval of the manuscript; completion of surgeries and/or experiments.
KM Completion of surgeries and/or experiments.
CI Final approval of the manuscript.
VFC Statistical analyses; conception and design of the study; writing the manuscript or critical review of its contents.
DSG Final approval of the manuscript.
RG Final approval of the manuscript.
REFERENCES
1. Blakey CM, Alexander KS, Galea G, Stewart KJ. The implications of a new Code of Practice on the storage of human skin for a regional plastic surgery unit. Burns. 2007;33(3):399-400. DOI: http://dx.doi.org/10.1016/j.burns.2006.08.005
2. Wentscher J. A further contribution about the survivability of human epidermal cells. Dtsch Z Chir. 1903;70:21-44. DOI: http://dx.doi.org/10.1007/BF02790822
3. Carrel A. The preservation of tissues and its applications in surgery. 1912. Clin Orthop Relat Res. 1992;(278):2-8. DOI: http://dx.doi.org/10.1001/jama.1912.04270080205010
4. Matthews DN. Storage of skin for autogenous grafts. The Lancet. 1945;245(6356):775-8. DOI: http://dx.doi.org/10.1016/S0140-6736(45)90544-7
5. Titley OG, Cooper M, Thomas A, Hancock K. Stored skin--stored trouble? Br J Plast Surg. 1994;47(1):24-9. PMID: 8124562
6. Bashaw MA. Guideline Implementation: Autologous Tissue Management. AORN J. 2015;102(3):270-80. DOI: http://dx.doi.org/10.1016/j.aorn.2015.07.003
7. Schiozer W. Banco de pele no Brasil. Rev Bras Queimaduras. 2012;11(2):53-5.
8. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada - RDC Nº 32, de 11 de junho de 2012. Dispõe sobre as diretrizes para embalagens primárias utilizadas no acondicionamento de tecidos humanos para fins terapêuticos e dá outras providências. Brasília: Ministério da Saúde; 2012.
9. Neves JR, Francesconi F, Costa A, Ribeiro BM, Follador I, Almeida LMC. Propionibacterium acnes e a resistência bacteriana. Surg Cosmet Dermatol. 2015;7(3 Supl 1):S27-38. DOI: http://dx.doi.org/10.5935/scd1984-8773.2015731683
10. Santos AL, Santos DO, Freitas CC, Ferreira BLA, Afonso IF, Rodrigues CR, Castro HC. Staphylococcus aureus: visitando uma cepa de importância hospitalar. J Bras Patol Med Lab. 2007;43(6):413-23. DOI: http://dx.doi.org/10.1590/S1676-24442007000600005
11. Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27(4):870-926. DOI: http://dx.doi.org/10.1128/CMR.00109-13
12. Van Wicklin SA, Brubaker SA, Conner R. Guideline for Autologous Tissue Management. In: 2015 Guidelines for Perioperative Practice. Denver: Association of peri Operative Registered Nurses (AORN); 2014. p. 187-238.
13. Li Z, Overend C, Maitz P, Kennedy P. Quality evaluation of meshed split-thickness skin grafts stored at 4ºC in isotonic solutions and nutrient media by cell cultures. Burns. 2012;38(6):899-907. DOI: http://dx.doi.org/10.1016/j.burns.2012.02.002
1. Instituto Israelita de Ensino e Pesquisa Albert Einstein, São Paulo, SP, Brazil
2. Universidade de São Paulo, São Paulo, SP, Brazil
Institution: Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.
Corresponding author:
Renata Oliveira da Conceição
Avenida Dr. Arnaldo, 455
São Paulo, SP, Brazil - Zip Code 01246-903
E-mail: renata.oliveira@hc.fm.usp.br
Article received: March 6, 2017.
Article accepted: September 23, 2017.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter