
Review Article - Year 2018 - Volume 33 -
Split-thickness skin graft donor-site dressings: is it possible to establish the ideal dressing based on a literature review?
Curativos tópicos para áreas doadoras de enxertos de pele parcial: é possível estabelecer o mais adequado com base em uma revisão da literatura?
ABSTRACT
This study aimed to assess the possibility of establishing the most suitable split-thickness skin graft donor site dressings on the basis of scientific evidence gathered through a literature review. The most relevant studies originally published in any language in the last 7 years and indexed in the US National Library of Medicine (PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), and Latin American and Caribbean Literature Health Sciences (LILACS) databases were evaluated. A literature survey was performed using keywords related to the theme and inclusion and exclusion criteria. The final sample comprised 25 publications, one domestic and 24 international. The results showed a gap in the literature with respect to studies that evaluated different split-thickness skin graft donor site dressings. The literature review revealed the impossibility of establishing the most effective split-thickness skin graft donor site dressing due to the lack of scientific evidence, thus preventing the formulation of a definite conclusion on this topic.
Keywords: Plastic surgery; Skin transplantation; Autologous transplantation; Wound Injury; Wound healing.
RESUMO
O objetivo deste estudo foi verificar, por meio de uma revisão da literatura, a possibilidade de se estabelecer, com base em evidências científicas, o curativo tópico mais adequado para a aplicação em áreas doadoras em enxertos de pele parcial. Foram analisados os mais relevantes estudos publicados originalmente nos éltimos sete anos, em qualquer idioma, porém, que estivessem indexados às bases de dados US National Library of Medicine (PubMed), Cochrane Central Register of Controlled Trials (CENTRAL) e Literatura Latino-Americana e do Caribe em Ciências da Saéde (LILACS). As buscas foram realizadas por meio do uso de descritores associados ao tema e de critérios de inclusão e exclusão. A amostra final deste estudo foi composta por 25 publicações, sendo uma nacional e 24 internacionais. Com base nos achados, constatou-se que há uma lacuna na literatura acerca de estudos que visam analisar os diferentes tipos de curativos usados em áreas doadoras em enxertos de pele parcial. Por meio da revisão da literatura realizada, pode-se concluir que não é possível se estabelecer o curativo mais adequado para uso em áreas doadoras de enxertos de pele parcial, devido à falta de evidências científicas que possibilitem um achado conclusivo acerca do tema.
Palavras-chave: Procedimentos cirérgicos reconstrutivos; Transplante de pele; Transplante autólogo; Ferimentos e lesões; Cicatrização.
Partial-thickness skin grafts are created using a reconstructive technique that offers many benefits, including accelerating the healing of burns, trauma, ulcers, and other wounds and reducing the occurrence of extensive scars1-8. In this context, well-established techniques are available for managing the skin graft locations to ensure a proper result and promote wound healing. However, a similar consensus does not exist with regard to the most appropriate care or donor site dressing to be applied that involves better healing and aesthetic acceptance9,10.
The partial-thickness skin collection process involves excision of the epidermis and part of the dermis, which leaves a wound in the donor area. Although such wounds are created under controlled and sterile conditions, they can be a considerable challenge for patients during and after the healing process because they cause itching, pain, infection, and aesthetic discomfort9.
These areas of partial-thickness skin graft donors generally receive healing dressings to assist with maintaining three main functions, namely patient comfort, scarring, and protection8. Succinctly, the ideal bandage must promote healing and be comfortable for the patient, impervious to infectious organisms, easy to handle, and low-cost10.
Dressings date back to prehistorical times, when they were prepared using poultices of leaves and herbs to stop bleeding and facilitate healing. Over time, various types of treatments have been implemented. In the nineteenth century, after knowledge was gained about the relationship between bacteria and infections, the aseptic concept in healing techniques was introduced.
Until World War II, emphasis was placed on the use of antiseptics and dressing agents with a dry cover when the question was raised about the toxicity of antiseptics and the introduction of antibiotics into dressings. Thereafter, bandages became sterile, followed aseptic techniques, and used hydrocolloid- and hydropolymer-based covers and transparent and porous films made of a wide range of materials11.
In short, the dressing must have some properties such as the following: 1) made of natural or artificial biocompatible and cytocompatible materials; 2) reduces risks of disease transmission and inflammatory and immune responses; 3) supports and stimulates cell migration owing to its optimized architecture; 4) retains moisture from the wound; 5) stabilizes the wound bed; and 6) supports quick healing with good aesthetic results5,6,8-10,12.
However, given the many dressings now available in the market and the low number of efficacy studies, which bandage shows the best performance before its application to donor areas of partial-thickness skin grafts is not yet known.
The aim of this study was to verify the possibility of identifying the topical bandage that is better suited for use in the donor areas of partial-thickness skin grafts, using scientific evidence extracted from a literature review.
METHODS
Research Strategy
To comply with the proposed objective, we analyzed the most relevant studies originally published in any language before or during July 2017 as long as they were indexed in the US National Library of Medicine (PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), and Latin American and Caribbean Health Sciences Literature (LILACS) databases.
To select studies with sufficient scientific evidence, we sought publications relating to meta-analyses and randomized controlled trials (RCTs) in humans. The publication period of 2011 to July 2017 was established as an inclusion criterion to ensure the inclusion of recent and current studies.
In the search procedure, we used the following combinations of keywords: "enxerto de pele parcial," "enxerto de pele," "área doadora," "região doadora," "curativo," and "cicatrização." The following terms of equivalence in English were used during the search in the international databases: "skin graft," "partial-thickness," "split-thickness," "donor site," "dressing," "management," and "treatment."
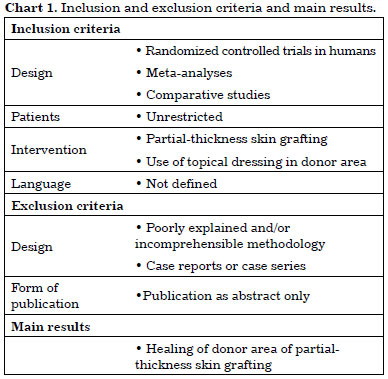
The inclusion and exclusion criteria were applied as shown in Chart 1.
RESULTS
No meta-analyses on the subject were found in the literature search. The final sample was composed of 25 RCT publications (Chart 2), one Brazilian study in the LILACS database and 24 international publications in the PubMed database. No studies were found in the CENTRAL database.
Chart 3 presents the main data related to the studies included in this analysis.
DISCUSSION
Considering the care needed in the donor areas of partial-thickness skin grafts, learning more about the dressings that can be applied in such wounds is necessary to provide correct maintenance that might lead to successful skin grafting and higher patient quality of life.
Therefore, we examined the possibility of choosing the most appropriate topical bandage for application in partial-thickness skin graft donor areas by conducting a literature review of studies that compared different approaches4,7,29-31 but did not include case reports or case series because they are unable to describe the clinical success (or lack thereof) of a specific dressing.
A considerable variety of issues analyzed in different studies was found. Pain referred by the patient, which is the most often analyzed factor, was featured in all but two studies6,16, of which one16 examined patient comfort instead. The second most often analyzed factor was re-epithelization/scarring, which appeared in all but three studies8,20,24; however, no other item correlated with it. Therefore, the differentiation of other items randomly analyzed by different studies hindered our ability to aggregate our findings and form an evidence-based conclusion.
A question of the studies that seem to be somehow standardized between them consisted of the donor region, for which the thigh was one of the locations used in all studies that specified where the partial-thickness skin samples were removed. However, this statement cannot be considered conclusive considering the number of studies that did not specify this aspect in their methodologies1,3,16,21,24.
It should be noted that in addition to the diversification between the points examined by the studies, follow-up time also showed a considerable variation, ranging from 1 day24 to 180 days1,8,18,21,28.
Nevertheless, one of the questions that most severely hampers the establishment of an ideal dressing for donor areas of partial-thickness skin grafts consists of the different approaches to applying dressings to the patients in different studies. Half of the studies2,4,5,9,10,14,15,18,20,23,24,28 (n = 12) used samples in which the patient received only one type of bandage.
This kind of approach is prone to creating biases that make data aggregation impossible because patients will respond according to the dressing applied. Therefore, one could raise the question that the results of these searches are not specifically related to the effects of the tested dressings themselves but rather to the influence generated by the specific organism to which it was applied. In other words, comparing the effects of a kind of bandage used in "John" with another type of bandage used in "Mary" is irrelevant because not only will the differentiation of dressings influence the results but also the distinction between organisms "John" and "Mary" will differ.
However, we must report that some studies used approaches that can be regarded as having greater credibility and less bias. The first is the approach used by some studies1,12,27,30 that used samples in which the number of donor areas used in the same patient is compatible with the number of dressings tested, causing the same patient to receive different dressings in different donor areas.
The second approach, which was adopted in eight studies3,6,8,13,17,19,25,26, used samples in which the same donor area in the same patient was divided between the number of dressings. Thus, these searches eliminated the bias caused by the differentiation of organisms in which the bandages were tested.
However, taking into account the objective of this research, the greatest difficulty related to the choice of an ideal dressing to be applied in the donor areas of partial-thickness skin grafts is associated with the diversity of the dressings available in the market, which are distinguished by not only the composition and active ingredients but also the trademarks and manufacturers.
In relation to the relevant literature, this situation is not different, as the publications reviewed in this research evaluated various dressings, both those that are commercially available and those that are not yet available but are being presented to academic, scientific, and professional communities, such as those by Khorasani et al.6, Assadian et al.12, Malin et al.23, Raza et al.24, Hasatsri et al.26, Salehi et al.27, and Subrahmanyam10.
There was also considerable technical-related diversity. Although most consisted of knitwear, fibers, and films1-3,5,8,9,12-18,20-22,25,26,28, some prefabricated dressings maintain humidity with individual substances through catheters24, feature gauze impregnated with different compounds6,10,19,27, or even consist of electronic devices23.
In general, in all the studies analyzed, 50 types of different dressings were examined, ranging from active products and trademarks, and the repetition of these bandages between the different studies was minimal, including Aquacel Ag® in four studies1,3,12,18; paraffin-impregnated gauze in four studies5,9,19,26 but under different trademarks (Adaptic®, Jelonet®, and Bactigras®); calcium alginate fiber in four studies9,15,17,22 under two distinct trademarks (Algisite® and Kaltostat®); and DuoDERM® hydrocolloid in three studies9,16,28. Therefore, this diversity of active ingredients and brands used in studies collaborates considerably to impede comparative credibility and confidence.
Finally, we found a gap in the literature of studies aimed at analyzing the different types of dressings used in donor areas in partial-thickness skin grafts. However, the establishment of future studies might be insufficient. Attention should be paid to the standardization methodologies and credibility, which can be used in different surveys with different patients to meet this demand.
Conducting new research around the topic is justified, primarily because of no literary consensus has been reached about the best dressing, which leaves surgeons at the mercy of their own experience or those of more experienced surgeons. Therefore, new research can assist in the decision-making process for surgeons to ensure that it is scientifically grounded.
CONCLUSION
On the basis of the literature review described here, we conclude that it is not possible to identify the most suitable dressing for use in donor areas of partial-thickness skin grafts in terms of comfort, scarring, aesthetic, and protective aspects because although studies demonstrated good results for different dressings, consensus is lacking about whether one is superior to the others.
COLLABORATIONS
RVER Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
OJDM Final approval of the manuscript; writing the manuscript or critical review of its contents.
REFERENCES
1. Bailey S, Carmean M, Cinat M, Burton K, Lane C, Malinoski D. A randomized comparison study of Aquacel Ag and Glucan II as donor site dressings with regard to healing time, cosmesis, infection rate, and patient's perceived pain: a pilot study. J Burn Care Res. 2011;32(6):627-32. DOI: http://dx.doi.org/10.1097/BCR.0b013e31822dc409
2. Dhanraj P. A Clinical Study Comparing Helicoll with Scarlet Red and OpSite in the Treatment of Split Thickness Skin Graft Donor Sites-A Randomized Controlled Trial. Indian J Surg. 2015;77(Suppl 2):385-92. DOI: http://dx.doi.org/10.1007/s12262-013-0850-3
3. Dornseifer U, Lonic D, Gerstung TI, Herter F, Fichter AM, Holm C, et al. The ideal split-thickness skin graft donor-site dressing: a clinical comparative trial of a modified polyurethane dressing and aquacel. Plast Reconstr Surg. 2011;128(4):918-24. PMID: 21681125 DOI: http://dx.doi.org/10.1097/PRS.0b013e3182268c02
4. Fanti PA, Dika E, Vaccari S, Misciali C, Ismaili A, Barisani A, et al. Repair of the donor areas defects after split-thickness skin grafts utilizing an advanced epithelialization dressing. J Dermatolog Treat. 2014;25(5):434-7. DOI: http://dx.doi.org/10.3109/09546634.2012.757286
5. Jorge JLG, Naif C, Marques EGSC, Andrade GAM, Lima RVKS, Müller Neto BF, et al. Malha de algodão parafinado versus malha de fibra de celulose salinizada como curativo temporário de áreas doadoras de pele parcial. Rev Bras Queimaduras. 2015;14(2):103-8.
6. Khorasani G, Ahmadi A, Jalal Hosseinimehr S, Ahmadi A, Taheri A, Fathi H. The Effects of Aloe Vera Cream on Split-thickness Skin Graft Donor Site Management: A Randomized, Blinded, Placebo-controlled Study. Wounds. 2011;23(2):44-8.
7. Konstantinow A, Fischer TV, Ring J. Effectiveness of collagen/oxidised regenerated cellulose/silver-containing composite wound dressing for the treatment of medium-depth split-thickness skin graft donor site wounds in multi-morbid patients: a prospective, non-comparative, single-centre study. Int Wound J. 2017;14(5):791-800. DOI: http://dx.doi.org/10.1111/iwj.12698
8. Schulz A, Depner C, Lefering R, Kricheldorff J, Kästner S, Fuchs PC, et al. A prospective clinical trial comparing Biobrane(®) Dressilk(®) and PolyMem(®) dressings on partial-thickness skin graft donor sites. Burns. 2016;42(2):345-55. DOI: http://dx.doi.org/10.1016/j.burns.2014.12.016
9. Brölmann FE, Eskes AM, Goslings JC, Niessen FB, de Bree R, Vahl AC, et al.; REMBRANDT study group. Randomized clinical trial of donor-site wound dressings after split-skin grafting. Br J Surg. 2013;100(5):619-27. DOI: http://dx.doi.org/10.1002/bjs.9045
10. Subrahmanyam M. Honey Dressing Accelerates Split-Thickness Skin Graft Donor Site Healing. Indian J Surg. 2015;77(Suppl 2):261-3. PMID: 26730006 DOI: http://dx.doi.org/10.1007/s12262-012-0789-9
11. Prefeitura Municipal de Florianópolis. Protocolo de cuidados de feridas. Florianópolis: Secretaria Municipal de Saéde; 2008.
12. Assadian O, Arnoldo B, Purdue G, Burris A, Skrinjar E, Duschek N, et al. A prospective, randomised study of a novel transforming methacrylate dressing compared with a silver-containing sodium carboxymethylcellulose dressing on partial-thickness skin graft donor sites in burn patients. Int Wound J. 2015;12(3):351-6. DOI: http://dx.doi.org/10.1111/iwj.12136
13. Kaartinen IS, Kuokkanen HO. Suprathel(®) causes less bleeding and scarring than Mepilex(®) Transfer in the treatment of donor sites of split-thickness skin grafts. J Plast Surg Hand Surg. 2011;45(4-5):200-3. PMID: 22150140 DOI: http://dx.doi.org/10.3109/2000656X.2011.583515
14. Muangman P, Nitimonton S, Aramwit P. Comparative clinical study of Bactigras and Telfa AMD for skin graft donor-site dressing. Int J Mol Sci. 2011;12(8):5031-8. DOI: http://dx.doi.org/10.3390/ijms12085031
15. Higgins L, Wasiak J, Spinks A, Cleland H. Split-thickness skin graft donor site management: a randomized controlled trial comparing polyurethane with calcium alginate dressings. Int Wound J. 2012;9(2):126-31. DOI: http://dx.doi.org/10.1111/j.1742-481X.2011.00867.x
16. Solanki NS, Mackie IP, Greenwood JE. A randomised prospective study of split skin graft donor site dressings: AWBAT-D™ vs. Duoderm®. Burns. 2012;38(6):889-98. DOI: http://dx.doi.org/10.1016/j.burns.2011.12.022
17. Davidson A, Jina NH, Marsh C, Than M, Simcock JW. Do functional keratin dressings accelerate epithelialization in human partial thickness wounds? A randomized controlled trial on skin graft donor sites. Eplasty. 2013;13:e45.
18. Ding X, Shi L, Liu C, Sun B. A randomized comparison study of Aquacel Ag and Alginate Silver as skin graft donor site dressings. Burns. 2013;39(8):1547-50. DOI: http://dx.doi.org/10.1016/j.burns.2013.04.017
19. Hassanpour SE, Moosavizadeh SM, Yavari M, Hallaj Mofrad HR, Fadaei A. Comparison of three different methods of dressing for partial thickness skin graft donor site. World J Plast Surg. 2013;2(1):26-32.
20. Healy C, Greig AV, Murphy AD, Powell C, Pinder RJ, Saour S, et al. Prospective randomized controlled trial: fibrin sealant reduces split skin graft donor-site pain. Plast Reconstr Surg. 2013;132(1):139e-46e. PMID: 23806933 DOI: http://dx.doi.org/10.1097/PRS.0b013e318299c6f4
21. Kaiser D, Hafner J, Mayer D, French LE, Läuchli S. Alginate dressing and polyurethane film versus paraffin gauze in the treatment of split-thickness skin graft donor sites: a randomized controlled pilot study. Adv Skin Wound Care. 2013;26(2):67-73. DOI: http://dx.doi.org/10.1097/01.ASW.0000426715.57540.8d
22. Läuchli S, Hafner J, Ostheeren S, Mayer D, Barysch MJ, French LE. Management of split-thickness skin graft donor sites: a randomized controlled trial of calcium alginate versus polyurethane film dressing. Dermatology. 2013;227(4):361-6. PMID: 24281776 DOI: http://dx.doi.org/10.1159/000356122
23. Malin EW, Galin CM, Lairet KF, Huzar TF, Williams JF, Renz EM, et al. Silver-coated nylon dressing plus active DC microcurrent for healing of autogenous skin donor sites. Ann Plast Surg. 2013;71(5):481-4. DOI: http://dx.doi.org/10.1097/SAP.0b013e31829d2311
24. Raza MS, Nazim T, Khan FA. Comparison of bupivacaine moistened dressing and conventional dressing for pain relief on skin graft donor sites. J Coll Physicians Surg Pak. 2014;24(6):416-9.
25. Tanaka K, Akita S, Yoshimoto H, Houbara S, Hirano A. Lipid-colloid dressing shows improved reepithelialization, pain relief, and corneal barrier function in split-thickness skin-graft donor wound healing. Int J Low Extrem Wounds. 2014;13(3):220-5. DOI: http://dx.doi.org/10.1177/1534734614541544
26. Hasatsri S, Angspatt A, Aramwit P. Randomized Clinical Trial of the Innovative Bilayered Wound Dressing Made of Silk and Gelatin: Safety and Efficacy Tests Using a Split-Thickness Skin Graft Model. Evid Based Complement Alternat Med. 2015;2015:206871. PMID: 26221170 DOI: http://dx.doi.org/10.1155/2015/206871
27. Salehi SH, As'adi K, Mousavi SJ, Shoar S. Evaluation of Amniotic Membrane Effectiveness in Skin Graft Donor Site Dressing in Burn Patients. Indian J Surg. 2015;77(Suppl 2):427-31. PMID: 26730039 DOI: http://dx.doi.org/10.1007/s12262-013-0864-x
28. Akita S, Yoshimoto H, Tanaka K, Oishi M, Senju C, Mawatari S, et al. Silver Sulfadiazine-Impregnated Hydrocolloid Dressing Is Beneficial in Split-Thickness Skin-Graft Donor Wound Healing in a Small Randomized Controlled Study. Int J Low Extrem Wounds. 2016;15(4):338-43. DOI: http://dx.doi.org/10.1177/1534734616670988
29. Hakkarainen T, Koivuniemi R, Kosonen M, Escobedo-Lucea C, Sanz-Garcia A, Vuola J, et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J Control Release. 2016;244(Pt B):292-301. DOI: http://dx.doi.org/10.1016/j.jconrel.2016.07.053
30. Millan L, Silva D, Coltro P, Almeida P, Mattar C, Faiwichow L. Curativo da área doadora de enxerto de pele parcial com curativo de colágeno e alginato (Fibracol®): uma experiência de 35 pacientes. Rev Bras Cir Plást. 2015;30(2):273-6.
31. Rocha FS, Simão TS, Pinheiro RR, Moscon FB, Barbosa FEAS, Almeida PCC, et al. Utilização de curativo de espuma de poliuretano e silicone (Mepilex Transfer®) em áreas doadoras de enxerto de pele parcial. Rev Bras Queimaduras. 2012;11(2):97-9.
1. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
2. Santa Casa de Montes Claros, Montes Claros, MG, Brazil
3. Hospital Dilson Godinho, Montes Claros, MG, Brazil
Institution: Santa Casa de Montes Claros, Montes Claros, MG, Brazil.
Corresponding author:
Rafael Vilela Eiras Ribeiro
Avenida Presidente Itamar Franco, 4001 - Dom Bosco
Juiz de Fora, MG, Brazil - Zip Code 36033-318
E-mail: vilelaeiras@hotmail.com
Article received: August 30, 2017.
Article accepted: November 27, 2017.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter