

Ideas and Innovation - Year 2018 - Volume 33 -
An autonomous infuser for a subcutaneous skin expander
Um infusor autônomo para um expansor de pele subcutâneo
ABSTRACT
Introduction: Skin expansion is a physiological process
defined as the ability of human skin to increase its
superficial area in response to a stress or given deformation.
In reconstructive surgery, skin expanders are implanted
beneath the skin and periodically infiltrated with a saline
solution to provide an extra flap of skin. When the prescribed
internal volume of the expander is reached, reconstructive
surgery is performed.
Methods: A semiautomatic and
portable device was developed and built to facilitate a
skin expansion procedure. The device aims to simplify the
infiltration process, providing mobility and independence
to the patient and assuring the physician of the infiltration
quality and precision. The device also enables continuous
expansion in hospitalized patients.
Results: Using a code,
the doctor accesses the menu of the device and sets the
maximum pressure and/or value for each expander of the
patient. The patient can control the infiltration velocity
and reverse or stop the operation. All data are recorded
on a simcard and include date, time, initial and final
volumes, and initial and final pressures of each procedure
for each expander.
Conclusions: The device motorizes
and optimizes the expansion, allowing the doctor to
prescribe a maximum infiltration pressure or volume. All
data are recorded to provide an important database of
skin behavior related to sex, race, age, and expansion site.
Keywords: Tissue expansion devices; Tissue expansion; Industrial development; Ambulatory surgical procedures; Mammaplasty
RESUMO
Introdução: A expansão da pele é um processo fisiológico definido como a capacidade de
aumentar sua área superficial em resposta a uma tensão ou a uma dada
deformação. Para realizar a cirurgia reconstrutiva, os expansores de pele
são implantados sob a pele e periodicamente infiltrados com uma solução
salina para fornecer um retalho extra de pele. Quando o volume interno
prescrito do expansor é alcançado, a cirurgia reconstrutiva é realizada.
Métodos: Foi desenvolvido um dispositivo semiautomático e portátil para facilitar um
procedimento de expansão da pele. O dispositivo tem como objetivo
simplificar o processo de infiltração, proporcionando mobilidade e
independência para o paciente, e assegurando ao médico a qualidade e a
precisão das infiltrações realizadas. O dispositivo também permite expansão
contínua em pacientes hospitalizados.
Resultados: Usando um código, o médico tem acesso ao menu do dispositivo e define a
pressão máxima e/ou o valor máximo para cada expansor do paciente. O
paciente pode realizar a infiltração e ter acesso ao controle da velocidade
de infiltração, reverter ou parar a operação. Todos os dados são gravados em
um SIM Card e incluem data, hora, volumes inicial e final, e pressão inicial
e final de cada procedimento para cada expansor.
Conclusões: O dispositivo automatiza e otimiza a expansão, de modo que o que o médico
possa prescrever um limite para cada expansão, seja uma pressão máxima ou
voluma infiltrado. Todos os dados são gravados, fornecendo um importante
banco de dados sobre o comportamento de pele relacionado a gênero, raça,
idade e local da expansão.
Palavras-chave: Dispositivos de expansão de tecido; Expansão de tecido; Desenvolvimento industrial; Procedimentos cirúrgicos ambulatoriais; Mamoplastia
INTRODUCTION
Skin expansion is a physiological process defined as the ability of human skin to increase its superficial area in response to stress or to a given deformation. Skin expanders are silicon prostheses of different shapes and sizes that are implanted underneath the skin. Because the skin exhibits creep or relaxation, the resulting stress decreases after a specific amount of time due to an imposed deformation. The physiology of skin expansion not only consists of the stretching of skin but also includes the relaxation process involved to obtain an extra flap of skin with specific characteristics.
Since the study conducted by Austad & Rose1, several authors such as Schmidt et al.2 have attempted to improve the expansion process using self-inflating or continuous and controlled expansions, such as Duffy & Shuter3. Several authors have also examined concepts and complications involved in the surgical process, and there have been numerous studies describing the findings associated with skin expansion from a medical perspective. However, few studies have investigated the bioengineering process.
A question that arises during skin expansion is with regards to the pressure inside the expander, which is important for determining if infiltration should proceed. Presently, the doctor does this intuitively.
When monitoring the internal pressure and injected volume during several skin expansions, Pamplona & Mota4 observed discrepancies in expansion when the prosthesis is located over fatty tissues. To monitor the skin expansion and check and identify the behaviour of the skin resulting from successive skin expansions, it was necessary to measure the pressure inside the skin expander before, during, and after injection of the saline solution for each expansion5-7.
OBJECTIVE
To study the process of skin expansion, a portable liquid infiltration device was designed and constructed that can record the relationship between the internal pressure and the volume of the fluid infiltrated during skin expansion as well the monitoring of internal pressure over time. This semiautomatic system aims to assist the surgeon in the skin expansion procedure, as well as enabling the process to be performed by the patient, following the doctor’s guidelines.
Thus, a patient in remote locations and with limited access to electricity can perform the procedure semi-autonomously, eliminating the need for periodic outpatient clinic attendance. The acquisition of the data from the expansion will allow several subsequent studies: the examination of the decrease of pressure from infusions in subcutaneous implants over time, the generation of data to identify the viscoelastic parameters of the skin, the determination of infiltration limits, the optimization of expanders and implants, and much more.
METHODS
The device has an easy-to-carry and clean design protected by a carrying case, which contains all the accessories required for use, as shown in Figure 1. Its operation features a user-friendly, touch- sensitive interface for both patient and physician use. In addition, throughout the infiltration process the user can control the pressure, maximum flow and volume and has the capacity to stop the procedure at any moment.
Only the doctor can adjust the settings and change the date and time; these are password-protected. The doctor enters settings for each expander, to a maximum of 5, for the maximum volume and pressure of each skin expansion as well as the maximum total volume to be infiltrated. The process automatically stops when the pressure reaches a critical value or when the total volume of infiltrated fluid is reached. The peristaltic pump can both infiltrate and withdraw liquid from the expander.
The patient uses the screen shown in Figure 2 to begin each infusion, selecting the skin expander to be infiltrated, as this operation can be performed using several expanders. The screen shows the actual pressure inside the expander, and the patient can control the flow of the infiltration and is able to stop the process at any point.
The data for each expansion are recorded separately in “.csv” files to relate pressure and volume with the date and time. The pump is easily sterilized via a removable, autoclave-resistant pump head, and the peristaltic pump does not come into contact with the liquid.
RESULTS
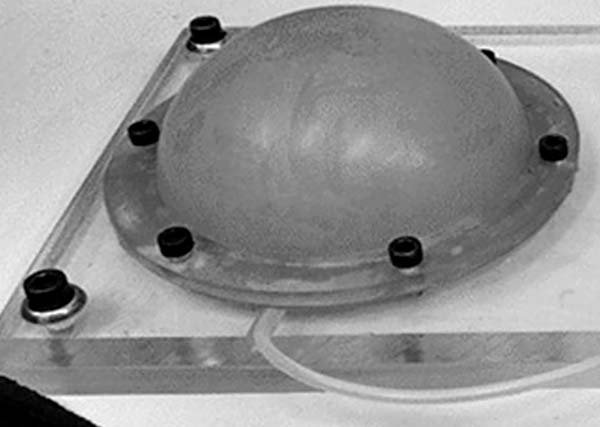
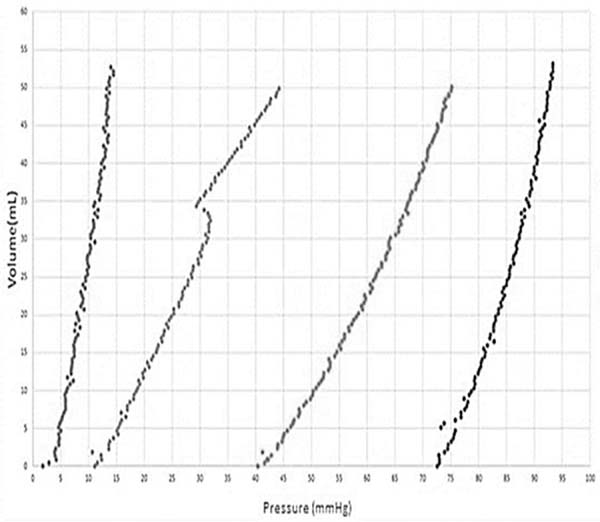
To simulate the skin expansion process, an elastic membrane was used to simulate the skin over a 200 mL round skin expander, as shown in Figure 3, which in turn is attached on the acrylic platform. Using the device with four infiltrations containing volumes of approximately 50 mL on four consecutive days, the internal pressure vs. the internal volume in the skin expander for each expansion, as shown in Figure 4.
From this figure it can be observed in the transition over days, the internal pressure inside the expander decreases; it is believed to be due to the viscoelastic properties of the membrane, which relaxes over time and which is much less than that of the skin. Note that in the second expansion a different behaviour is observed, where a dip in pressure, followed by a curve that does not follow the previous trend. This may be due to the skin expander adjusting itself over the acrylic base.
DISCUSSION
A proprietary design was developed in a waterproof case for portability, safety and ease of handling with the ability to determine the pressure and volume, display them on the touchscreen interface, and save them on a removable MicroSd card, recording the date and time for as many as five expanders. In addition, the device is powered by a rechargeable lithium-ion battery and uses a programmable microcontroller.
CONCLUSIONS
To improve the skin expansion process, a portable liquid infiltration device was designed and constructed that can record the relationship between the internal pressure and the volume of the fluid infiltrated during the skin expansion as well as monitoring the internal pressure over time. This semiautomatic system can assist the surgeon in the skin expansion procedure, enabling the process to be performed by the patient. Thus, a patient in a remote location and with little access to electricity can perform the procedure.
The device motorizes and optimizes the expansion since the doctor can prescribe a limit to each expansion for either the maximum pressure or the volume infiltrated. All data are recorded, which provides an important and precise database regarding skin behaviour for bioengineering medical studies relating to gender, race, age and expansion site. The device successfully allows a new way to perform skin expansion due to its portability and vast storage capacity.
Acknowledgements
We are grateful to late Professor Ivo Pitanguy and his staff for the support given to our projects over the years.
COLLABORATIONS
|
DCP |
Analysis and/or data interpretation, conception and design study, conceptualization, funding acquisition, investigation, methodology, project administration, realization of operations and/ or trials, resources, supervision, validation, writing - original draft preparation, writing - review & editing. |
|
RCP |
Conception and design study, conceptualization, data curation, investigation, realization of operations and/or trials. |
|
HIW |
Analysis and/or data interpretation, investigation. |
|
GRS |
Methodology, realization of operations and/ or trials. |
|
HNR |
Conceptualization, final manuscript approval. |
REFERENCES
1. Austad ED, Rose GL. A self-inflating tissue expander. Plast Reconstr Surg. 1982;70(5):588-94.
2. Schmidt SC, Logan SE, Hayden JM, Ahn ST, Mustoe TA. Continuous versus conventional tissue expansion: experimental verification of a new technique. Plast Reconstr Surg. 1991;87(1):10-5.
3. Duffy JS, Shuter M. Evaluation of soft-tissue properties under controlled expansion for reconstructive surgical use. Med Eng Phys. 1994;16(4):304-9.
4. Pamplona DC, Motta DEJS. Numerical and experimental analysis of inflating a circular hyperelastic membrane over a rigid and elastic foundation. Int J Mech Sci. 2012;65(1):18-23.
5. Pamplona DC, Carvalho CR. Characterization of human skin through skin expansion. J Mech Mater Struct. 2012;7(7):641-55.
6. Pamplona DC, Velloso RQ, Radwanski HN. On skin expansion. J Mech Behav Biomed Mater. 2014;29:655-62.
7. Pamplona DC, Weber HI, Leta FR. Optimization of the use of skin expanders. Skin Res Technol. 2014;20(4):463-72.
1. Pontifícia Universidade Católica do Rio de
Janeiro, Rio de Janeiro, RJ, Brazil.
2. Instituto Ivo Pitanguy, Rio de Janeiro, RJ,
Brazil.
Corresponding author: Djenane Cordeiro Pamplona, Departamento de Engenharia Mecânica da PUC-Rio, Rua Marquês de São Vicente nº 225 - Gávea - Rio de Janeiro, RJ, Brazil, Zip Code 22451-900. E-mail: djenane@puc-rio.br
Article received: May 17, 2018.
Article accepted: October 4, 2018.
Conflicts of interest: none.







 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter