

Special Article - Year 2019 - Volume 34 -
Plastic surgery for the treatment of contagious diseases: lobomycosis
Cirurgia plástica e o tratamento de doenças infectocontagiosas: lobomicose
ABSTRACT
Introduction: Lacaziosis is a rare disease that mainly affects workers in tropical areas, with approximately 500 cases reported worldwide. Lacaziosis is a parasitic disease caused by the saprophytic fungus Lacazia loboi; there is no specific treatment for this disease. Surgery is the most effective treatment for the deformities caused by the disease. However, it is a temporary treatment, since disease recurrence is frequently observed. Lacazia loboi affects two species of dolphin, Tursiops truncates and Sotalia guianensis. The available literature discusses the surgical treatment in a superficial way , because there are no specific studies describing the surgical treatment for this disease.
Methods: Here, we describe our 8 years of experience with lacaziosis at the Hospital de Base de Porto Velho - Rondônia; a total of 22 patients underwent surgical treatment and were followed-up.
Results: The majority of the patients (91%) had already submitted to at least one surgical treatment together with antifungal treatment. The patients presented with lesions with disease progression ranging from 5 months to 6 years prior to surgical treatment. Only two patients were treatment-naive.
Conclusion: Our patients were followed-up; however, only 11 of the 22 patients returned for follow-up. Recurrences were observed in 9 of the 11 patients, with a latency period of 5 months.
Keywords: Lobomycosis; Reconstructive surgical procedures; Communicable diseases; Advanced treatment; Recurrence; Lacazia
RESUMO
Introdução: Lacaziose é uma doença rara que afeta principalmente trabalhadores de áreas tropicais, sendo descritos aproximadamente 500 casos no mundo. A lacaziose é um doença parasitária causada pelo fungo saprófita Lacazia loboi, para o qual não existe um tratamento específico. A cirurgia é o tratamento mais eficiente para as deformidades causadas pela doença. Entretanto, é um tratamento temporário, uma vez que as recidivas são frequentes. Lacazia loboi acomete duas espécies de golfinhos, o Tursiops truncates e o Sotalia guianensis. A literatura aborda o tratamento cirúrgico de maneira superficial, pois não existem trabalhos específicos descrevendo o tratamento cirúrgico para essa doença.
Métodos: Descrevemos aqui nossos 8 anos de experiência no Hospital de Base de Porto Velho-Rondônia com 22 casos submetidos a tratamento cirúrgico e acompanhados.
Resultados: A maioria dos pacientes (91%) já se submeteram a pelo menos um tratamento cirúrgico associado ao tratamento antifúngico. Os pacientes apresentavam lesões com tempo de evolução entre 5 meses e 6 anos previamente ao tratamento cirúrgico. Apenas dois casos eram virgens de tratamento.
Conclusão: Nossos pacientes foram acompanhados, mas apenas 11 dos 22 pacientes retornaram para acompanhamento. Recorrências foram observadas em 9 dos 11 pacientes, com um período de latência de 5 meses.
Palavras-chave: Lobomicose; Procedimentos cirúrgicos reconstrutivos; Doenças transmissíveis; Tratamento avançado; Recidiva; Lacazia
INTRODUCTION
The first report of Lobo’s disease, called lobomycosis or currently known as lacaziosis, was first described in 1930 by a Brazilian dermatologist, Jorge Lobo.
The majority of the cases described in humans, approximately 500 cases worldwide, is restricted to jungle regions, with hot and humid climate, abundant water courses, and high rainfall, such as the Brazilian Amazon region.
Approximately 300 cases were registered in Brazil; lacaziosis cases have also been reported in Peru, Colombia, Venezuela, French Guiana, Guyana, Bolivia, Ecuador and Suriname, with approximately 200 registered cases registered1; rare cases have also been described in other parts of the world, such as North America2,3, Central America and some European 4,5 and African countries6.
The etiologic agent is the saprophytic fungus, Lacazia loboi, which is present in water, soil and vegetation. The mode of transmission is not known, and infection may be transmitted by inoculation of the fungus by solutions of continuity of skin; this is often caused by trauma with plant fragments and insect stings7,8.
History and pathogenesis
The parasite, Lacazia loboi, over time, has been designated in different ways, with the current nomenclature being adopted in 1999 by Taborda et al.7. The term lacazia comes from the name of the Brazilian mycologist, Carlos da Silva Lacaz, who greatly contributed to a better understanding of this disease, and the term loboi was adopted to honor the first physician to describe the disease, Jorge Lobo.
In 2007, Hibbett et al.8 classified the fungus as belonging to the phylum Ascomycota, subphylum Pezizomycotina, class Eurotiomycetes, subclass Eurotiomycetidae, order Onygenales, and family Onygenceae.
Its phylogenetic classification was based on the identification of the fungus using the 18s ribosomal subunit DNA and a 600-bp fragment of the CHS2 gene (chitin synthetase 2) from yeast cells 9,10.
In addition to humans, two species of dolphin, Tursiops truncates11 and Sotalia guianensis12.13, are affected by this parasite.No cases of lacaziosis in harbor porpoises or dolphins in the area of the Amazon basin have been described.
The individuals most affected are laborers in the aforementioned areas, with most of them being rubber tappers, lumberjacks, farmers, fishermen, and miners of precious stones7,8; these individuals are usually males aged around 50 years14.
Little is known about the mode of transmission of the disease; the only known way is the inoculation of the parasite from cells infected with the fungus. Inter-human transmissions have never been described. There is only one report of transmission to humans in the literature, wherein the infection was transmitted by a piercing and blunt accident with material infected with the parasite15,16; however, transmission may occur when there is contamination in areas of the skin with loss of continuity (wounds) and lobomycosis lesions11.
Once in contact with the dermis, the fungus is phagocytosed and slow proliferation of the parasite is then initiated. Dissemination may occur through local lymphatic continuity via the regional and hematogenic ganglia7,16,17. There is no information regarding the incubation period or latency of this disease; however, elimination or treatment of this infection is difficult due to the high resistance of the parasite, with disease recurrence being very frequent. There are reports of individuals infected by dolphins, with the latency periods varying from 3 months to 4 years18-20.
There is only one case report of lacaziosis11. The immunological response of the skin to the parasite is still not clearly understood. From a histological viewpoint, the lesions are characterized by little organized granulomas composed of histiocytes (CD68), Langerhans cells, and multinucleated giant cells. Immunohistochemical studies revealed the presence of a small number of the following mononuclear cells: T lymphocytes (CD3+), helper T lymphocytes (CD4+), cytotoxic T lymphocytes (CD8+), B lymphocytes (CD20+), plasmocytes (CD79+), and NK cells (CD57+)19. Vilani-Moreno et al.21 suggested that fungi are phagocytosed by histiocytes, which give rise to the giant Langerhans cells; thus, the typical granulomas observed in this disease are formed.
Langerhans cells are responsible for the presentation of antigens in numerous infections. To better understand the disease, Quaresma et al.18 performed an immunohistochemical study of Langerhans cells and verified that although there are no morphological changes with respect to normal cells, there is an escape mechanism for the presentation of these antigens by Langerhans cells21.
The is no association between lobomycosis and specific antigens of the class II HLA system; however, the decrease in the frequency of HLA-DR7 antigen in the patient group compared to the control group indicates a protective relationship of HLA-DR7 with lobomycosis.
Clinical Presentation
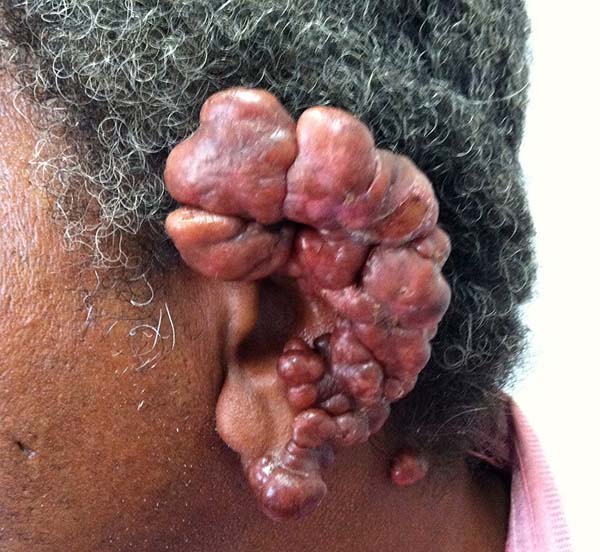
The lesions of lobomycosis usually appear in exposed areas and in response to trauma; the lesions are frequently observed in the ears, upper and lower limbs, face, chest, and cervical region, and especially in areas with low temperature.
The clinical diagnosis of lobomycosis is usually delayed, since the patients seek medical service due to a nodular lesion in the subcutaneous tissue that is slightly pruriginous and painless and grows slowly and steadily over the years.
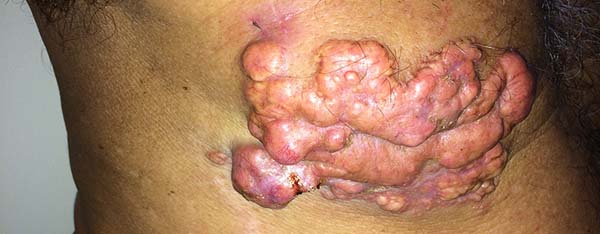
Brito & Quaresma6 classified the lesions of lobomycosis as monomorphic and polymorphic (macules, papules, nodules, gums, nodular plaques, verruciform lesions, scarring, and aphthous lesions), with a predominance of nodular lesions.
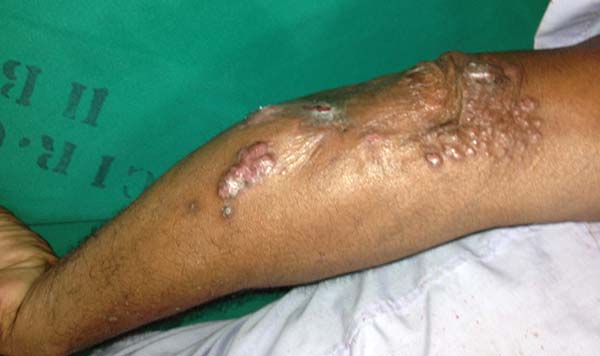
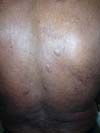
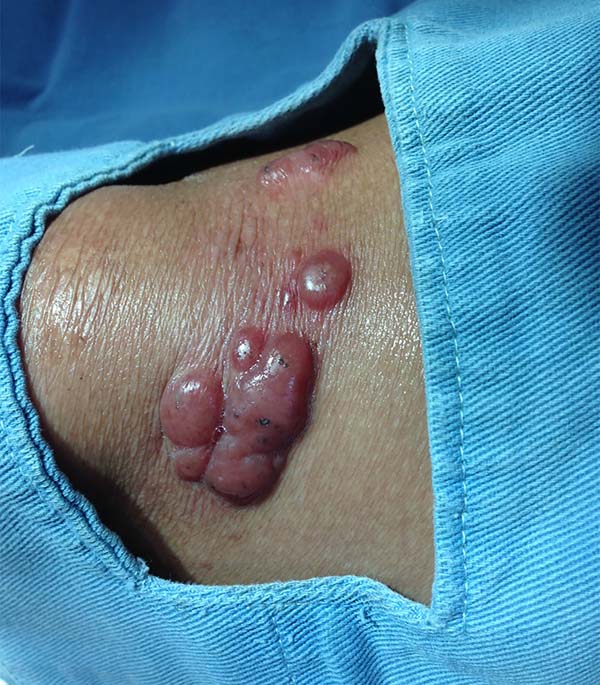
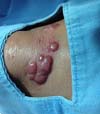
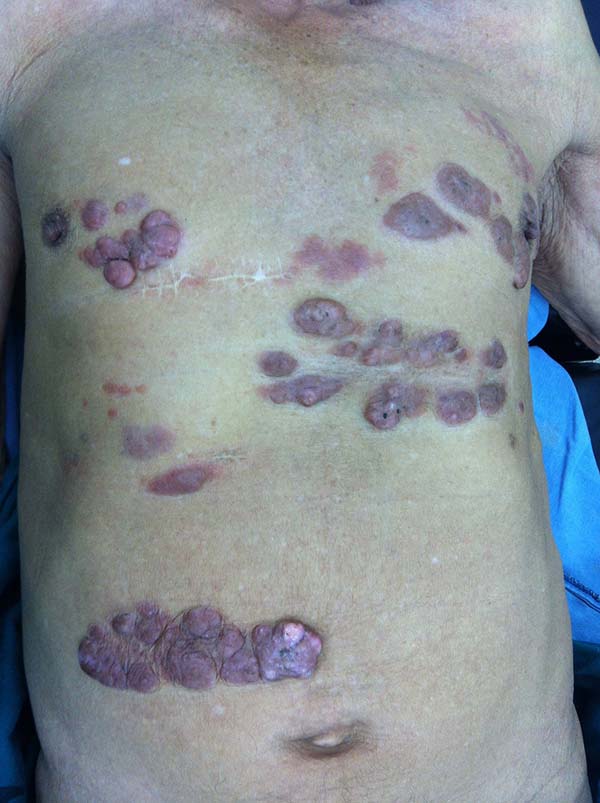
More recently Opromolla et al.20, ranked them as: i) isolated form (Figure 1); (ii) disseminated form (Figure 2); and (iii) multifocal form (Figure 3), that is, multiple localized lesions in just one limb or limb segment. There are other descriptions based on the morphological appearance of the lesions, such as descriptions provided by Lacaz14, Dias et al.22, and Ramos and Silva23, which are as follows: infiltrative, keloidal, plaque, verruciform, and ulcerated (Figures 4-6, respectively). Another lacaziosis classification system was suggested by Lacaz24 et al., who described it as isolated form, disseminated form, and multifocal (Figures 4, 5, and 6, respectively). The existing classifications often differ only in the description of the lesions (author’s note).
Diagnosis
The diagnosis can be established by mycological examination, histopathological assessment, and immunohistochemical staining. To date, the culture of this parasite has not been obtained13. In our service, the mycological examination is conducted initially through puncture of the nodules, aspiration of the contents, and direct observation of the parasite on a slide. The anatomopathological examination is performed with hematoxylin-eosin staining, as proposed by Vilela et al.10. Fungi can also be visualized by fresh Grocott-Gomori staining or with calcofluor and Periodic acid–Schiff (PAS) staining7.
Staining with ethidium bromide revealed that the majority of the fungi is non-viable, since ethidium bromide is a marker of nucleic acids, and the parasites found do not have nucleic acids in the cell membrane21. The cells are yeast-like, and spherical, with a diameter ranging from 6 µm to 12 µm; the cells have birefringent cell membranes and may be isolated or may show gemmulation, usually presenting with a rosary distribution.
The epidermis can be normal, atrophic, and hyperplasic or ulcerated with fungi that are located in the hyperplastic bottom25. The evaluation of the dermis infiltrates of lymphocytes, histiocytes, epithelioid cells, giant cells, plasma cells, and eosinophils, revealed vasodilation and vascular neoformation. The histiocytes present sporadically with fungi in their interior, suggesting that they are phagocytosing the fungi; these cells eventually give rise to the multinucleated giant Langerhans cells26.
Differential Diagnosis
There are several conditions that may interfere with the diagnosis of lobomycosis or lacaziosis. These include the more frequently observed keloid scar27 (Figure 7), other fungal infections, such as cromomycosis (Figure 8), sporotrichosis, phaeohyphomycosis, and histoplasmosis, tuberculoid leprosy (Figure 9), verruciform elephantiasis resulting from paracoccidioidosis (Figure 10), and diffuse anemia leishmaniasis (Figure 11).
A well-performed clinical history is of paramount importance for the differential diagnosis, especially in case of diseases associated with fungal infections, since the clinical evolution of this type of disease is usually slow, and confusion may arise due to the association with other events experienced by the patient throughout life.
Treatment
To date, there is no effective treatment for lacaziosis. Several studies have reported on the surgical treatment and disease relapse, but little is known regarding the best technique that can be used with respect to the lesion margins, involvement of other tissues, and the time to disease recurrence.
Currently the choice of treatment is defined according to the clinical presentation of the disease. For the unifocal and localized forms, surgical treatment is performed, followed by treatment with various combinations of medications, such as clofazimine (50 mg/day), dapsone (100 mg/day), or itraconazole15, which begins immediately after surgery and is continued for at least one year, with the aim to decrease the chances of relapse and eventually achieve a cure.
According to Miranda et al.27,28, administration of clofazimine in the postoperative period at doses of 300 mg/day in the first month, 200 mg/day in the second month, and 100 mg/day until completing 24 months of treatment best impedes disease relapse. Some reports suggested that the disease was cured with the use of posaconazole29 and of other drug combinations7.
The best results in the medium term are obtained with surgical treatment, since it devolves a little of the quality of life of patients who present with deforming injuries and invoke revulsion30.
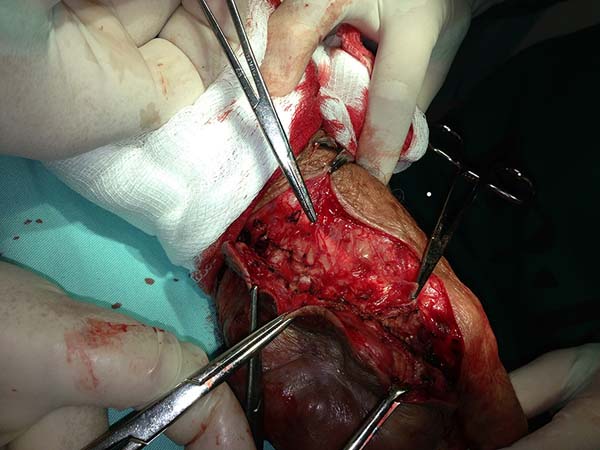
Surgical treatment
Several reports describe the surgical treatment of lacaziosis; however, there are only a few studies on the techniques used during the surgical treatment of these lesions. Here, we will discuss the surgical aspects of the lesions of the patients submitted to surgical treatment at the Hospital de Base de Porto Velho and Hospital Santa Marcelina from June 2006 to July 2014.
Informed Consent
All patients submitted to surgical treatment signed the informed consent form authorizing the surgery, and anatomopathological assessment and allowed the dissemination of data regarding the treatment used and the photographs taken during the course of treatment. This study is registered with the institutional Ethics Committee under number 48872115.9.0000.0013.
METHODS
From 2006 to 2014, a total of 22 patients, 19 male and 3 female, underwent surgical treatment. The patients were aged 35–68 years. None of the patients had relatives with the disease or similar lesions, confirming the findings reported by Dias et al. in 197022.
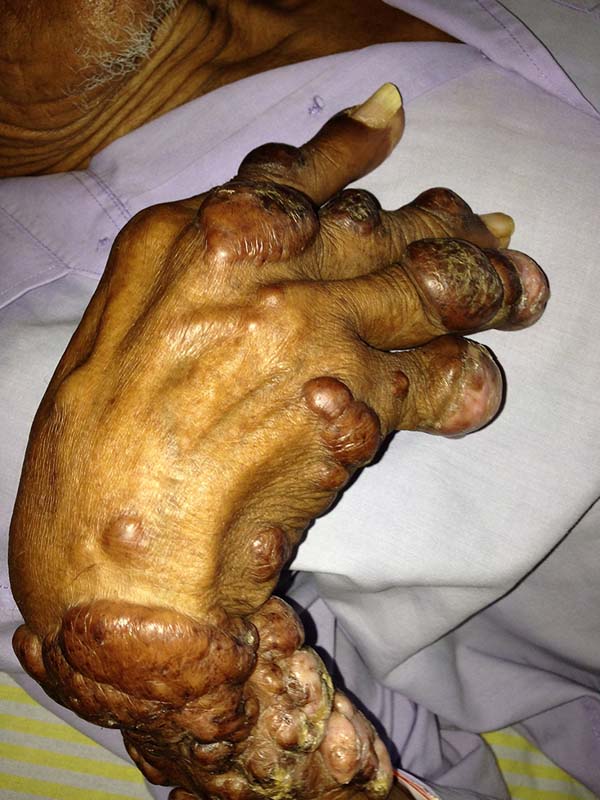
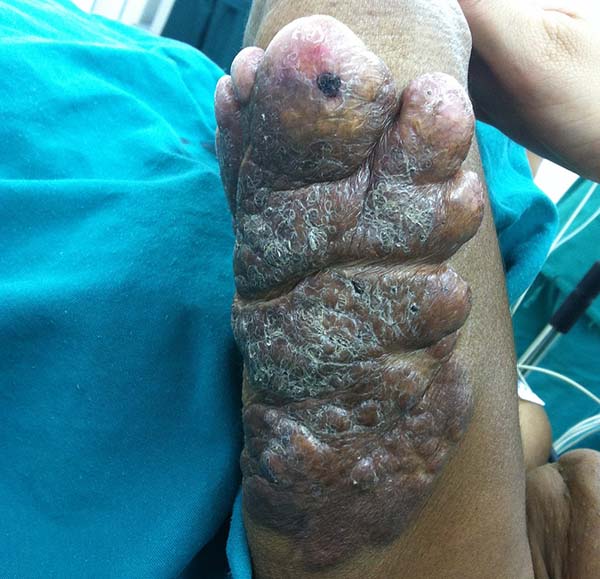
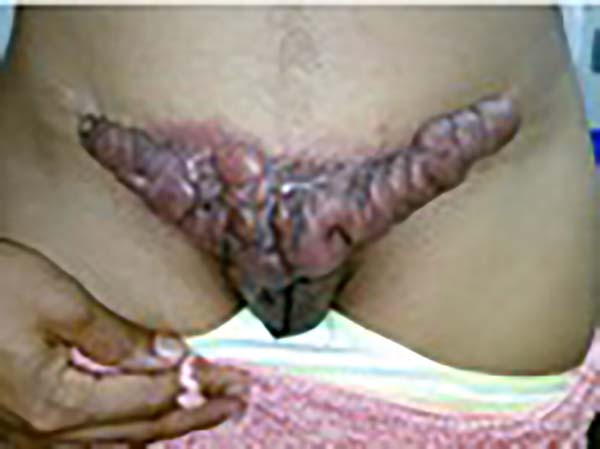
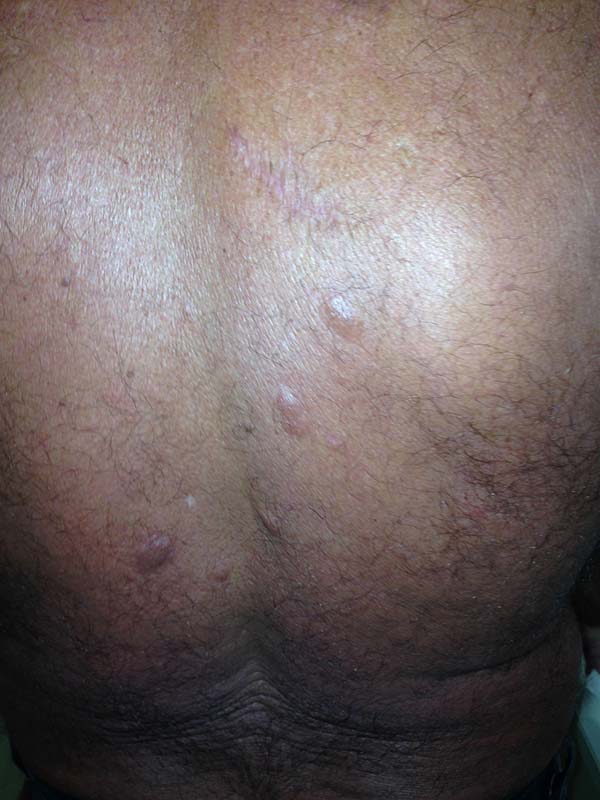
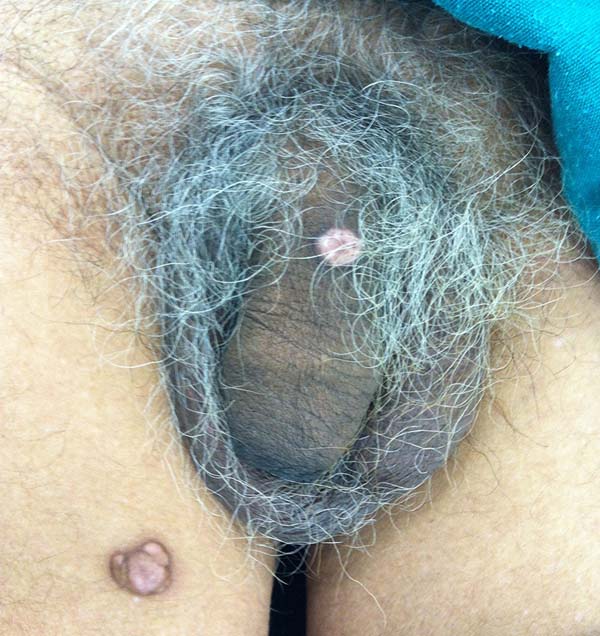
A 97-year-old patient diagnosed with the disease 63 years ago was referred to our clinic for the removal of lesions on the trunk (Figure 12), hands, and penis (Figure 13); he had previously undergone resection of some lesions and was not aware of the precise number of previous surgeries. He also underwent drug treatments, without mentioning which.
He did not allow surgeons to remove the lesions that bothered him. This patient had disseminated lobomycosis, with numerous lesions on the thorax, hands, arms, legs, and an injury to the body of the penis.
This patient returned 5 years later, with the same complaints and presented with slight clinical evolution of the nodulations. He did not allow surgical treatment again; however, the case drew our attention due to the slow evolution of the pre-existing lesions.
Among the 22 patients who submitted to surgical treatment, the duration of the disease ranged from 7 years to 45 years.
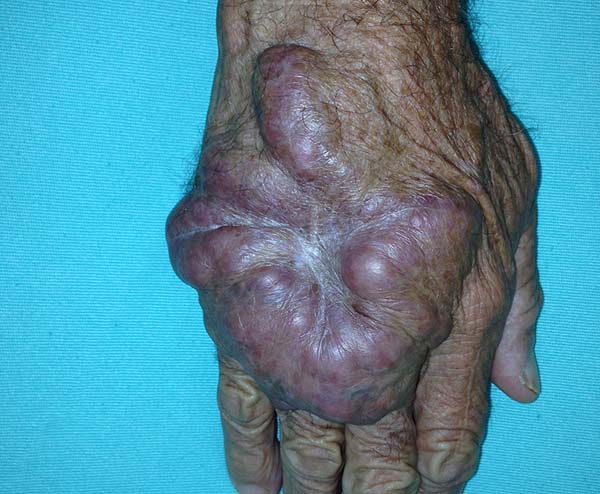
The clinical presentation in these patients was as follows: the lesion was located at only 1 site in 9 patients; 10 patients, including 6 male patients, presented with lesions in only 1 ear and 1 patient presented with a lesion in the middle third of the right thigh; 3 female patients presented with a lesion in the right arm, while another presented with a scar on the left lumbar region, and 1 patient presented with a popliteal cavity lesion; localized form with local dissemination and/or symmetrical (same injured anatomical structure) distribution was observed in 11 patients, with lesions being located in both ears in 3 patients and on the limbs in 7 patients with focal lesions on the hand, dorsum of the hand, forearm, with multiple nodules of lacaziosis, suggesting the occurrence of local dissemination, while 1 patient presented with a lesion in the foot with nodules in the dorsal region of the same foot; and 2 patients presented with the systemic form of lacaziosis, i.e., numerous disseminated lesions, one of whom refused surgical treatment.
All patients visited our facility with complaints of pruritic nodular lesions, and 20 of 22 patients had already undergone at least one surgery, with time of disease recurrence ranging between 5 months and 6 years. Two patients has not undergone any surgical and clinical treatment previously and presented with slow growing pruritic nodular lesions; they had developed the lesions following insect stings approximately 1.5 years ago.
Twenty-one patients were rural workers, and only 1 lived in the countryside, but it was of the home.
Surgery
All patients underwent clinical and laboratory assessment, prior to the surgery; the following tests were performed: blood workup, estimation of urea, creatinine, and blood glucose levels, coagulation tests, and electrocardiography. Patients who were administered local or regional anesthesia were evaluated by the anesthesia team.
Lesions in the ears
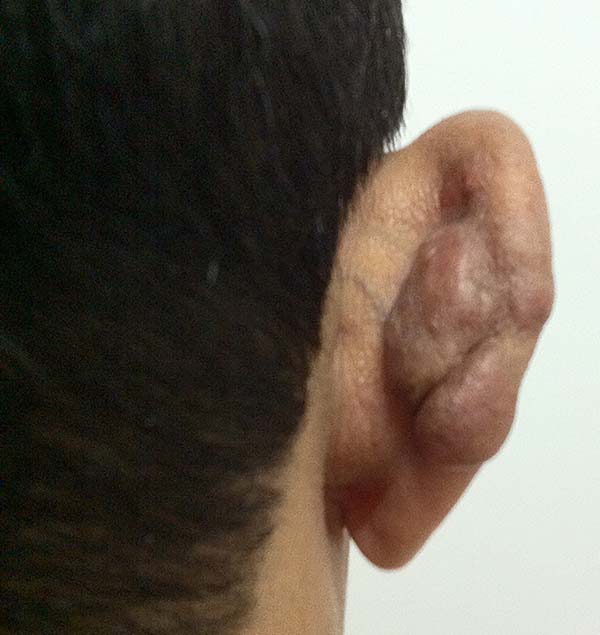
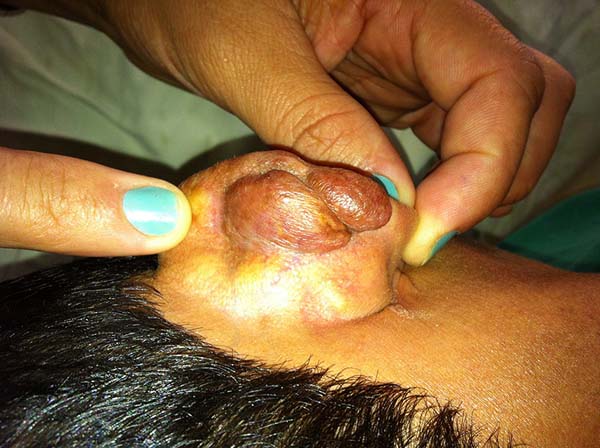
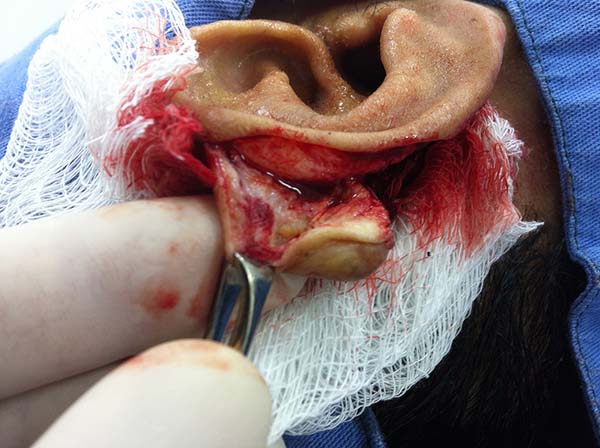
In patients with ear lesions, we were able to confirm that injuries do not invade the cartilage, affecting only the skin and subcutaneous tissue during the surgical treatment (Figures 14 and 15); this has already been reported in the available literature.
The cartilage in all cases had a normal macroscopic aspect. Among patients who had undergone surgical treatment of lesion in this regions, many presented with partial resection of the ear lesions, including the cartilage, being the withdrawal of existing lesions and the reconstruction of the pinna performed at the same surgical time, when possible.
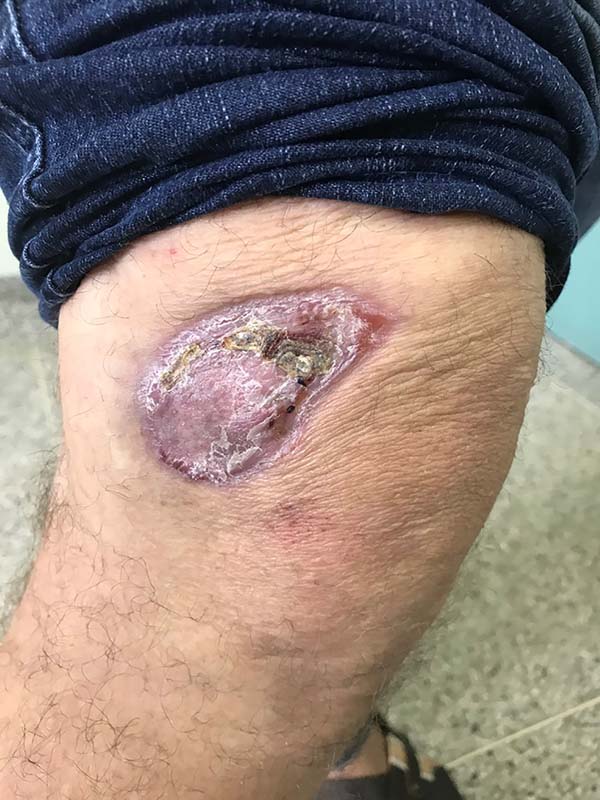
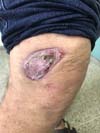
In patients with lesions in the forearm, the nodular lesions on the arm and hand were resected; these lesions were circumscribed lesions and did not invade the adjacent tissues, blood vessels, muscles, and tendons (Figure 16 and 17).
DISCUSSION
The patients who submitted to surgical treatment received no adjuvant treatment. Twenty of the 22 patients were already administered medication for lobomycosis, without any significant improvement. They refused medication due to the difficulty presented in receiving the medication, which is a prolonged treatment. In our 8-year experience of operating on and monitoring these patients, we noticed that disease relapse will occur, but there is no specific time for disease relapse. Among the 11 patients who returned to our facility for follow-up, 9 presented with disease relapse; the time of relapse ranged from 5 months to 6 years. The remaining patients, or are recent cases, or did not return for follow-up.
In many case, disease recurrence, such as small nodules and/or nodules along the affected limb, was noted on the grafted areas. Surgical margins were 0.5 cm of the lesion edge and the deep margin; the deeper tissues were not affected.
Lobomycosis is a deforming disease that deprives patients of social conviviality, and surgery is the only effective treatment for the removal of the lesions, although it is a temporary option. Patients are informed about relapses, and surgery is always the chosen option to relieve them of the discomfort caused by the presence of the lesions.
CONCLUSION
We confirm that surgery is one of the effective treatments for lacaziosis. All the characteristics described in the literature were confirmed, namely, the lesions did not affect the other tissues, such as blood vessels, tendons, and cartilage.
We could not confirm whether the dissemination route of the disease was lymphatic, hematogenic, or contiguity. We suggest that the disease recurrence occurred in the grafted areas because greater deep margin resection could not be performed without damaging the adjacent tissues.
Little is known about lacaziosis and its pathogenesis; it is a rare disease without an effective clinical treatment. Plastic surgery units should be responsible for the surgical treatment of this disease, which, when indicated, preserves noble structures, such as auricular cartilage, and restores the patients’ self-esteem and dignity, albeit temporarily.
COLLABORATIONS
|
RLK |
Analysis and/or data interpretation, conception and design study, final manuscript approval, investigation, methodology, realization of operations and/ or trials, supervision, visualization, writing - original draft preparation, writing - review & editing. |
|
CFJ |
Analysis and/or data interpretation, conception and design study, data curation, investigation, methodology, visualization, writing - review & editing. |
|
AEKC |
Data curation, investigation, realization of operations and/or trials, writing - review & editing. |
|
LFF |
Analysis and/or data interpretation, data curation, realization of operations and/or trials, writing - review & editing. |
|
ASP |
Analysis and/or data interpretation, data curation, investigation, validation. |
REFERENCES
1. Lupi O, Tyring SK, McGinnis MR. Tropical dermatology: fungal tropical diseases. J Am Acad Dermatol. 2005;53(6):931-51. DOI: https://doi.org/10.1016/j.jaad.2004.10.883
2. Burns RA, Roy JS, Woods C, Padhye AA, Warnock DW. Report of the first human case of lobomycosis in the United States. J Clin Microbiol. 2000;38(3):1283-5. PMID: 10699043
3. Symmers WS. A possible case of Lôbo's disease acquired in Europe from a bottle-nosed dolphin (Tursiops truncatus). Bull Soc Pathol Exot Filiales. 1983;76(5 Pt 2):777-84.
4. Saint-Blancard P, Maccari F, Le Guyadec T, Lanternier G, Le Vagueresse R. Lobomycosis: a mycosis seldom observed in metropolitan France. Ann Pathol. 2000;20(3):241-4.
5. Al-Daraji WI, Husain E, Robson A. Lobomycosis in African patients. Br J Dermatol. 2008;159(1):234-6. PMID: 18460023 DOI: https://doi.org/10.1111/j.1365-2133.2008.08586.x
6. Brito AC, Quaresma JAS. Lacaziose (doença de Jorge Lobo): revisão e atualização. An Bras Dermatol. 2007;82(5):461-74. DOI: https://doi.org/10.1590/S0365-05962007000500010
7. Taborda P, Taborda VA, McGinnis MR. Lacazia loboi gen. nov., comb. nov., the etiologic agent of lobomycosis. J Clin Microbiol. 1999;37(6):2031-3.
8. Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, et al. A higher-level phylogenetic classification of the Fungi. Mycol Res. 2007;111(Pt 5):509-47. PMID: 17572334
9. Herr RA, Tarcha EJ, Taborda PR, Taylor JW, Ajello L, Mendoza L. Phylogenetic analysis of Lacazia loboi places this previously uncharacterized pathogen within dimorphic Onygenales. J Clin Microbiol. 2001;39(1):309-14. DOI: https://doi.org/10.1128/JCM.39.1.309-314.2001
10. Vilela R, Mendoza L, Rosa OS, Belone AF, Madeira S, Opromolla DV, et al. Molecular model for studying the uncultivated fungal pathogen Lacazia loboi. J Clin Microb. 2005;43(8):3657-61. DOI: https://doi.org/10.1128/JCM.43.8.3657-3661.2005
11. Rosa PS, Soares CT, Belone Ade F, Vilela R, Ura S, Filho MC, et al. Accidental Jorge Lobo's disease in a worker dealing with Lacazia loboi infected mice: a case report. J Med Case Reports. 2009;3:67. DOI: https://doi.org/10.1186/1752-1947-3-67
12. Caldwell DK, Caldwell MC, Woodard JC, Ajello L, Kaplan W, McClure H. Lobomycosis as a disease of the Atlantic bottle-nosed dolphin (Tursiops truncatus Montagu, 1821). Am J Trop Med Hyg. 1975;24(1):105-14. PMID: 1111350 DOI: https://doi.org/10.4269/ajtmh.1975.24.105
13. Paniz-Mondolfi AE, Reyes Jaimes O, Dávila Jones L. Lobomycosis in Venezuela. Inter J Dermatol. 2007;46(2):180-5. DOI: https://doi.org/10.1111/j.1365-4632.2007.02937.x
14. Lacaz CS. Paracoccidioides loboi (Fonseca Filho et Arêa Leão, 1940) Almeida et Lacaz, 1948-1949: description of the fungus in latin. Rev Inst Med Trop Sao Paulo. 1996;38(3):229-31. DOI: https://doi.org/10.1590/S0036-46651996000300013
15. Paniz-Mondolfi AE, Sander-Hoffmann L. Lobomycosis in inshore and estuarine dolphins. Emerg Infect Dis. 2009;15(4):672-3. DOI: https://doi.org/10.3201/eid1504.080955
16. Elsayed S, Kuhn SM, Barber D, Church DL, Adams S, Kasper R. Human case of lobomycosis. Emerg Inf Dis. 2004;10(4):715-8. DOI: https://doi.org/10.3201/eid1004.030416
17. Borelli D. Lobomicosis experimental. Dermatol Venez. 1962;3:72-82.
18. Quaresma JA, Unger D, Pagliari C, Sotto MN, Duarte MI, de Brito AC. Immunohistochemical study of Langerhans cells in cutaneous lesions of the Jorge Lobo's disease. Acta Trop. 2010;114(1):59-62. DOI: https://doi.org/10.1016/j.actatropica.2009.12.005
19. Marcos EV, Souza FC, Torres EA, Lauris JR, Opromolla DV. Study of the association between human leukocyte antigens and Jorge Lobo's disease. Rev Soc Bras Med Trop. 2005;38(5):399-401. PMID: 16172755 DOI: https://doi.org/10.1590/S0037-86822005000500007
20. Opromolla DVA, Belone AFF, Taborda PRO, Taborda VBA. Correlação clinicopatológica em 40 novos caos de lobomicose. An Bras Dermatol. 2000;75(4):425-44.
21. Vilani-Moreno FR, Belone AF, Soares CT, Opromolla DV. Immunohistochemical characterization of the cellular infiltrate in Jorge Lobo's disease. Rev Iberoam Micol. 2005;22(1):44-9. DOI: https://doi.org/10.1016/S1130-1406(05)70006-1
22. Dias LB, Sampaio MM, Silva D. Jorge Lôbo's disease. Observations on its epidemiology and some unusual morphological forms of the fungus. Rev Inst Med Trop Sao Paulo. 1970;12(1):8-15.
23. Ramos-e-Silva M, Aguiar-Santos-Vilela F, Cardoso-de-Brito A, Coelho-Carneiro S. Lobomycosis. Literature review and future perspectives. Actas Dermosifiliogr. 2009;100 Suppl 1:92-100.
24. Lacaz CS, Porto E, Martins JEC, Heins-Vaccari EM, De Melo NT. Tratado de Micologia Médica Lacaz. 9ª ed. São Paulo: Sarvier; 2002. p. 23-8.
25. Miranda MF, Costa VS, Bettencourt Mde J, Brito AC. Transepidermal elimination of parasites in Jorge Lobo's disease. An Bras Dermato. 2010;85(1):39-43. DOI: https://doi.org/10.1590/S0365-05962010000100005
26. Queiroz LdS. Técnicas histológicas empregadas no departamento de anatomia patológica - FCM - UNICAMP. [Internet]; 2011 [cited 2016 Feb 5]. Available from: http://anatpat.unicamp.br/tecnicashistologicas.html
27. Silverie R, Ravisse P, Vilar JP, Moulins C. Keloid blastomycosis or Jorge Lobo disease in French Guiana. Bull Soc Pathol Exot Filiales. 1963;56:29-35. PMID: 13992935
28. Carneiro FP, Maia LB, Moraes MA, de Magalhães AV, Vianna LM, Zancanaro PC, et al. Lobomycosis: diagnosis and management of relapsed and multifocal lesions. Diagn Microbiol Infect Dis. 2009;65(1):62-4. DOI: https://doi.org/10.1016/j.diagmicrobio.2009.04.013
29. Bustamante B, Seas C, Salomon M, Bravo F. Lobomycosis successfully treated with posaconazole. Am J Trop Med Hyg. 2013;88(6):1207-8. DOI: https://doi.org/10.4269/ajtmh.12-0428
30. Korte RL, Feitosa LF, Porto AS, Ferreira Junior C, Closs J. Relato de caso de tratamento cirúrgico da lacaziose. Arq Catarin Med. 2014;43(Suppl 1):14-7.
1. Universidade Federal de Rondônia, Porto Velho,
RO, Brazil
2. Hospital de Base Ari Pinheiro, Porto Velho, RO,
Brazil
3. Centro De Medicina Tropical, Porto Velho, RO,
Brazil.
Corresponding author: Rodolfo Luis Korte Rua Anizio Gorayeb, nº 1331 - Porto Velho, RO, Brazil Zip Code 76803-680 E-mail: rlkorte@uol.com.br
Article received: May 5, 2018.
Article accepted: November 11, 2018.
Conflicts of interest: none.






 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter