

Articles - Year 2000 - Volume 15 -
Dorsal Decubitus in the Microsurgical Flap of Latissimus Dorsi - J. M. Servant Technique
Decúbito Dorsal no Retalho Microcirúrgico do Músculo Grande Dorsal - Técnica de J. M. Servant
ABSTRACT
100 microsurqical flaps were peiformed at the University Hospital of Paris VII - Hospital Saint-Louis and studied at the Federal University of São Paulo (UNIFESP) - Escola Paulista de Medicina (EPM) and Federal University of Alagoas (UFAL). The implementation of dorsal decubitus as preoperative position provided an option that eased the dissection of the flap and the performance of the surgery in a single position; the anesthetic team thus, could worl: with a definite position, which favored the fixation of the endotracheal tube and the monitoring of vital signs. Thank to these factors, the surgery was carried out with safety and comfort. The average of 9 surgery patients per year confirmed the tendency of not overestimating microsurqical flaps. There was no age limit that contraindicated microsurgery, which was carried out in patients with ages from 6 to 78 years old, with an average of 37.8 years. The areas of lower limbs and head were those which were most indicated for great dorsal microsurgical flap; the percentage of a new surgical intervention was 11% due to thrombosis; and total flap loss was 4%, all of them located at the lower limbs.
Keywords:
RESUMO
Foram realizados 100 retalhos microcirúrgicos no Hospital da Universidade de Paris VII - Hospital Saint-Louis e estudados na Universidade Federal de São Paulo (UNIFESP) - Escola Paulista de Medicina (EPM) e Universidade Federal de Alagoas (UFAL). A sistematização do decúbito dorsal como posição no pré-operatório propiciou uma opção que facilitou a dissecção do retalho e a realização da cirurgia numa só posição; a equipe de anestesia pôde, então, trabalhar com uma posição definitiva, o que favoreceu a fixação do tubo endotraqueal e a monitoração dos parâmetros clínicos. Graças a esses fatores, a cirurgia foi realizada com segurança e conforto.
A média de 9 pacientes operados por ano confirmou a tendência de não se supervalorizar os retalhos microcirúrgicos. Não houve limites de idade que contra-indicassem a microcirurgia, que foi realizada em pacientes de 6 e 78 anos, com média de 37,8 anos. As regiões dos membros inferiores e a da cabeça foram as que tiveram maior indicação do retalho microcirúrgico do grande dorsal; a porcentagem de reintervenção cirúrgica foi de 11% devida à trombose; e a perda total do retalho foi de 4%, todos localizados em membros inferiores.
Palavras-chave:
The Latissimus Dorsi Flap (LDF), first used in 1896(1) for the reconstruction of the thoracic area after mastectomy, had great acceptance in Europe in the beginning of the century, with the disclosure provided by a important essay in 1912(2). Its application in the reconstructive surgery had a new repercussion with the presentation of the rotation of a cutaneous-fascialmuscle flap in an island of the Large Dorsal Muscle (LD)(3)and with the description of two thoracodorsal microsurgical flap (MF) cases, in which the anterior segment of LD was included(4). Later on, a plurality of essays was published on the clinical application of LD(5).These authors presented a case of Latissimus Dorsi Microsurgical Flap (LDMF), with the patient in lateral decubitus, for the reconstruction and treatment of an osteomielitis with exposure of cranial parietals.
Currently, LDMF is widely used in plastic surgery, due to its little variable anatomy and its multiple applications(6,7,8). In microsurgery, LD is many times indicated for reconstructions that involve extremities, such as the vertex of the scalp and plantar face of the foot. During the individualization and rotation of LD, the intra-operative position of the patient is quite important. When positioning the patient in ventral or lateral decubitus for LD dissection, the transfer of the flap requires a change in the position during surgery, and it may increase surgical morbidity and time span. The possibility of using LD in dorsal decubitus (DR) avoids the change in the patient's position and creates an option for microsurgical reconstructions.
The present study aims at analyzing LDMF methods carried out with the patient in dorsal decubitus.
CASUISTIC AND METHOD
In this study, 100 LDMF cases were retrospectively evaluated at the University Hospital of Paris VII - Hospital Saint-Louis and studied at the Hospital São Paulo, Federal University of São Paulo (UNIFESP) - Escola Paulista de Medicina (EPM) and at the University Hospital of the Federal University of Alagoas (UFAL). All patients were registered by computerized and uniform clinical forms, 56% of the patients were males and 44% females, with ages raging from 6 to 78 years old (average 38 years old).
Preoperative and surgical procedures to individualize LDMFs and their placement in beds of the receptive region were similar in all cases. Certain precautions were important prior to LDMF surgical indication, such as carrying out a medical and previous radiation and/or surgical intervention history, both in the donor and receptive region(9). A spinal X-ray was also carried out in children, to be the standard for future evaluations(7). Since they were not considered as a priority(10), additional exams, such as arteriography and doppler, were not requested.
The dynamic evaluation of LD was carried out with the in patient sitting or standing position, with the arm in a forced adduction and hands over iliac crests.
The outlining of the medial limit was initiated with the muscle in contraction, aiming at visualizing and palpating LD's medial border(11). The rotational axis of the flap corresponds to the origin of vascular pedicle located at the center of the axillar fossa, with the arm being in complete abduction.
After outlining the flap and inhalatory anesthesia with endotracheal intubation, the patient was positioned in dorsal decubitus, with his arm abducted over an auxiliary hand surgery table support.
After that, in order to lift patient's lateral position, a longitudinal pad was placed (an approximately 30-cm long and 14-cm diameter large surgical drape bent over itself) along the paravertebral line, at the side to be operated.
The approach to the vascular-nervous pedicle was started with a reverse V-incision at the axillar fossa, with a medial apex and with an approximate 140º angle, which involved skin and subcutaneous tissue. After that, the incision was prolonged according to the desired shape and constitution of the flap. When the flap was a muscular one, the prolonging was longitudinal, along the middle axillar line. When a curaneous-fascial-muscle flap was used, the prolonging was along the medial outlining, observing the topography of the LD with thoracic and abdominal regions muscles. The incision of the cutaneous lateral segment was performed according to the desired width to cover the cutaneous defect, and started from the distal extremity going up to the proximal extremity, aiming at easing the approach to the pedicle region.
The blood vessels of the receptive region were prepared for microsurgical anastomosis, when the vascular- nervous pedicle was sectioned, after individualizing and releasing the flap, which was placed in a sterile container, with Ringer's lactate and heparin solution, at a dosage of two 5-ml ampoules, with 5000 U/ml in each 1000 ml of Ringer's lactate at room temperature. Afterwards, the apposition of the MF on the receptive region was performed, and the vascular sutures were performed at the arteries, with 8 to 10 separated stitches, 8-0 13-cm single-strand polyamide (nylon) black surgical threads, cylindrical steel 150µ diameter 0.6-cm needle; at the veins, the suture was carried out with eight separated stitches, with the same surgical threads. The vascular clamps used had a 30-60 g/mm pressure for arteries and veins, with 1 to 2-mm diameter.
In all cases, the suture of the donor area was performed with dermal supporting separated stitches, using synthetic absorbable threads, with glycolic acid 90% and lactic acid 10%, and 3/8 round, 3-cm long violet sharp needles,with a diameter of approximately zero.
In the cases of flaps with a cutaneous island smaller than 12cm, the skin was sutured with separated stitches, with single-strand black 2-0 polyamide threads (nylon), and a sharp needle.
During the immediate postoperative, integrity of the flaps was evaluated using color, digital pressure, temperature and distal dermal bleeding after puncture or scratches with needles as parameters.
In surgical cases involving the lower limb, this was kept lifted for three consecutive weeks.
In all cases, the dressing was made with uniform pressure over the flap margin, covered with open and cushioned gauze (dressing made with hydrophilic and hydrophobic cotton involved in fine gauze), and in the center of the flap an opening was left for visualization and monitoring, aiming at observation at the immediate postoperative period and strict clinical parameter follow-up during the next 3 days.
The vascular pedicle of the crossed MF, carried out in 7 patients, was temporarily anastomosed in blood vessels that were located far from the receptive area. This variation from the technique followed the crossed flap principles(12,13).
The technique in which the flap is bent over itself for a variable period (between 3 and 7 days) is good to demonstrate its vascular feasibility before the excision of the receptive region.
RESULTS
Table I - Number of MFs removed per year
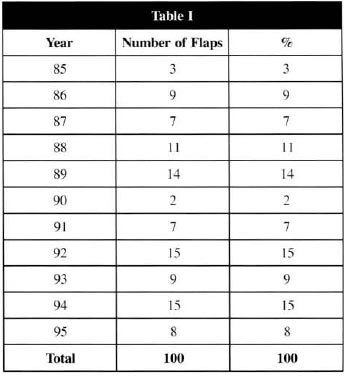
Table II - Receptive area of LDMF
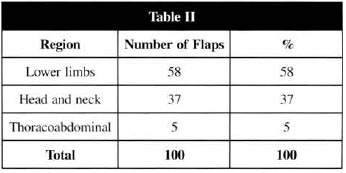
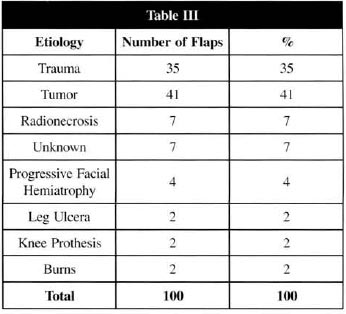
Table III - MFs distribution regarding the lesion ethiology
DISCUSSION
LD is one of the most important elements of the posterolateral thoracic wall and the topography of its lateral segment is quite important for its identification during surgery. In addition to being of great volume, it is wide but thin, preseming a triangula shape axillary apex(14), being used to cover defects that require great length and less muscular thickness.
LD is vascularized by the thoracodorsal artery and vein, descending branches of the subscapular artery and vein, that have an average length of 11 cm and low evidence of the presence of atheromatous accumulation (8%)(14). These characteristics are ideal for the performance of a MF.
In the MFs studied, the dissection of the LD was carried out with the patient in dorsal decubitus, according to other authors(11). Such fact was important in establishing the implementation of dorsal decubitus as an easy to be carried out position, when related with the other commonly used positions, such as lateral and ventral decubitus.
The use of dorsal decubitus allowed: the possibility of using two surgical teams, one working at the donor region of the flap and the other at the receptive area, which may help in reducing the intraoperative time; simplifies the operation, with more comfort for the surgeon, who may carry out all the dissection and hemostasis in a sitting position, since there are no changes in the decubitus in most of the cases; the suture of the donor area becomes easier, and it may be carried out by other team, while the surgeon may begin simultaneously the microsurgical anastomosis. The approach to the pedicle, which is short when it penetrates the LD, being located close to the anterior muscle margin, is eased with dorsal decubitus and with the arm narurally positioned in abduction. The anesthesia team may work with the patient in a definite position, not being necessary the change in decubitus and operative drapes, since the patient is anesthetized in dorsal decubitus and remains in the same position throughout the surgery, which eases the fixation of the endotracheal tube and monitoring clinical parameters.
All patients went through laboratorial and clinical evaluation, the surgery only being carried out in hemodinamically stable patients. Smoking patients were requested to quit smoking three weeks prior to the surgery. Authors report that smoking does not directly influence possible complications with MF(10). Auto-immune deficience patients and diabetic patients (Diabetes mellitus) underwent surgery only with their additional exams within normal ranges(8).
All patients with prior history of radiation therapy ar surgery in the region of the vascular pedicle of the donor area were excluded, since fibrosis, caused by radiation therapy, compromises the possibility of carrying out a MF, and the surgical approach to the pedicle region may damage this area. Arteriography and doppler were not considered important, since they would not bring benefits for the surgical indication and for the patient(7,15).
The spinal X-ray was considered important for specific cases, such as those which involved children, since they may cause a change in the frontal plan, reduction in the muscular strength and vertebral spine deviation, after releasing the flap. Thus, prior X-ray is a standard for future postural evaluations(7,16).
The relationship between LD with the fibers of the external oblique muscle is surgically important, more precisely over the external area of the posterior arch of the last four ribs, since, in this area, it is quite difficult to separate LD, and considering that this is a region where there is a major bleeding during surgery(17).
The analysis of the average of 9 patients operated per year, in the time span of 10 years of this 100-case series, led us to believe that there has not been an overestimate of the MFs indications by the services involved (Hospital Saint-Louis/Paris, France, Hospital São Paulo-Brazil and University Hospital/Maceió- Brazil), since the literature examples confirmed the said trend(8,10). Another point to be highlighted: there were no age limits that contraindicated microsurgery, and it has been carried out in patients aged from 6 to 78 years old (with a 37.8 years old average).
The suture of the fascial-cutaneous muscle flap donor area of LD is one of the reasons several students of the subject condemn the use of the flap with a large cutaneous island (larger than 12 cm, there is great difficulty in approximating the wound borders). Among the 100-patient series, 83 fascialcutaneous muscle flaps were carried out, and all of those with a cutaneous island smaller than 12 cm had their borders approximated by dermal supporting stitches(18).
For the lower limb and head areas, with the highest indication for LDMF, results were especially similar to those found in the literature(9,10,19). LDMF was most indicated in great losses of substances direct1y related to the structures of lower limbs and head, where it should be pointed that the fascial-cutaneous muscle flaps do not have the ability to solve certain problems, such as bane exposure or osteomielitis, that require blood supply that only a muscle can bring. It is also important to highlight that there are no pediculate flaps in the most distal areas of the human body that can caver great cutaneous losses.
The surgical anastomosis aimed at the maximum MF feasibility, and the veins chosen for the anastomosis were those that were not narrowed, the flexion areas being avoided, with patient in dorsal decubitus. By the way, the artery and vein of a LDMF may be anastomosed whenever necessary, due to the high blood output, aided by a saphenous vein bypass, or preferably, with veins from the forearm, since these veins have a lower number of valves, when compared with that vein(20). In large fascial-cutaneous flaps, we reduced the muscle around the pedicle, with the priority being the increase in the blood supply to the flap(12). In some particular cases, this procedure was carried out with good results. However, we believe that a randomized clinical trial may be necessary so as to define its efficiency, and a study with lab animals in order to confirm its vascular feasibility.
The percentage of reintervention was 11%, due to the occurrence of thrombosis(8,21). In 7% of the cases, there was a complete feasibility of the flap right after the surgery; the percentage of total flap loss was 4%, all of them at the lower limbs, 3% being due to traumatic ethiology. We believe that the small percentage of LDMF total loss is due to its characteristics, such as long and thick vascular pedicle (regarding other MFs), as well as its constant anatomy and limited occurrence of atheromatous accumulation in the vascular lumen of subscapular and thoracodorsal arteries(6,10,14,15,19).
At the lower limbs, by preference order, termino-terminal anastomoses were performed at the anterior and posterior tibial arteries and at the internal saphenous, anterior tibial, posterior tibial and external saphenous veins.
When there was no option to perform anastomosis in the blood vessels dose to recipient area, femoral blood vessels were used (10.7%), by means of termino-terminal anastomosis using saphenous vein bypass or forearm vein, so as to avoid thrombosis. When it was not possible to perform the anastomosis directly at the limb, the crossed MF method was performed. Currently, the crossed parascapular flap is mostly used, due to the need to sacrifice the LD, when using it as a erossed MF, since, when the section of the anastomosis pedicle is performed, the muscle will lose its blood supply and necrose. Possibly, the drawback of this erossed MF is that, after the anastomosis section, it will behave as a "parasite flap" (that has a blood supply that comes from the borders and bed of the receptive area) and will not have a blood supply as a LDMF, with direct anastomosis and blood supply. This may lead us to believe that its role in bone consolidations ean not be compared to a common MF 22).
For neck and head areas, the external carotid artery in termino-terminal (25%) and the branches from the external carotid artery in termino-terminal (75%) were used.
Preferably, the external jugular vein in termino-terminal (75%) and internal jugular vein in termino-terminal (25%) were chosen.
We believe that the temporal superficial artery and vein are not adequate as receptive vein and artery for a MF and, we have always adopted the jugular venous system and the external carotid artery branches (12).
In the bent flap technique, the microsurgical anastomosis was carried out; however, we should point out that the transposition of the flap was performed at the second operative stage, so as to visualize the blood supply, thus having more safety to transfer the fiap. Said procedure was only used in extreme cases, i.e., in specific cases where flaps were too large or those arisen from the general status of the receptive area or the patient was forced to choose this surgical technique. Thus, the excision was postponed so as to perform the flap apposition. The average 41-day hospitalization is consistent with the highly difficult procedures and the diseases involved.
Finally, we highlight that the surgical difficulty with the patient in dorsal decubitus did not have a comparing element; however, made this position feasible, since all surgical procedures were performed in dorsal decubitus and there was no complication strictly arisen from the surgical position.
CONCLUSION
The LDMF is feasible in the operative dorsal decubitus position of the patient.
REFERENCES
1. TANSINI I. Nuovo processo per I'amputazione della mammella per cancro. Riforma Med. 1896;1.
2. D'ESTE S. La technique de I'amputation de la mammelle pour carcinome mammaire. Rev. Chir. Orthop. Paris. 1912;45:164.
3. OLIVARI N. The latissimus dorsi flap. Brit. J. Plast. Surg. 1976;29:126-8.
4. BAUDET J, GUIMBERTEAU J, NASCIMENTO E. Successful clinical transfer of two free thoracodorsal axillary flaps. Plast. Reconstr. Surg. 1976;58:680-8.
5. MAXWELL GP, STUEBER K, HOOPES JE. A free latissimus dorsi myocutaneo flap. Plast. Reconstr. Surg. 1978;62:462-6.
6. LEGRE R, BARDOT O, KEVORKIAN B, VASSE D, EMRAM A, AUBERT JP, MAGALON G, BUREAU H. Evaluation of 106 free flaps. Analysis of the failures and the indications. Ann. Chir. Plast. Esthet. 1989;34:385-9.
7. LEGRE R, BOGHOSSIAN V, SERVANT JM. Analyse des séquelles du prélèvement du lambeau de grand dorsal (à propos de quarante quatre cas revus et testés) Ann. Chir. Plast. Esthet. 1990;35:512-7.
8. GLICKSMAN A, FERDER M, CASALE P, POSNER J, KIM R, STRAUCH B. 1457 Years of microsurgical experience. Plast. Reconstr. Surg. 1997;100:355-67.
9. HIDALGO DA, CARRASQUILO IM. The treatment of lower extremity sarcomas with excision, radiotherapy and free flap reconstruction. Plast. Reconstr. Surg. 1992;89:96-101.
10. KROLL SS, SCHUSTERMAN MD, MARK A, REECE MD, GREGORY P, MILLER MD, EVANS MD, GREGORY R, ROBB MD, GEOFFREY L, ROBB MB, BONNIE J, BALDWIN MD. Choice of Flap and incidence of Free Flap Success. Plast. Reconstr. Surg. 1996;98;459-63.
11. MOLE B, SERVANT JM, BANZET P. Le prélèvement du lambeau de grand dorsal en decubitus dorsal. Ann. Chir. Plast. Esthet. 1986;31:79-81.
12. REVOL M, VERGOTE T, SERVANT JM, BANZET P. Transferts tissulaires libres en chirurgie plastique (urgences exclues): A propos d'une experience de dix ans. Ann. Chir. Plast. Esthet. 1992;37:450-9.
13. DING SY. Treatment of chronic osteomyelitis of leg with free latissimus dorsi musculocutaneous flap anastomosed to contralateral leg vessels. Chung Hua Cheng Hsing Shao Shang Wai Ko Tsa Chih. 1993;9(2):106-7,159.
14. BARTLETT SP, MAY JW, YARENCHUK MJ. The latissimus dorsi muscle: a fresh cadaver study of the primary neurovascular pedicle. Plast. Reconstr. Surg. 1981;67:631-6.
15. HIDALGO DA, JONES CS. The role of emergent exploration in free tissues transfer: A review of 150 consecutive cases. Plast. Reconstr. Surg. 1990;492-8-86.
16. FRAULIN F, LOUIE G, ZORRILLA L, TILLERY W. Functional evaluation of the shoulder following latissimus dorsi muscle transfer. Ann. Plast Surg. 1995;35:349-55.
17. FRIEDRICH W, HERHOLD C, LIERSE W. Vascularization of the myocutaneous latissimus dorsi flap. Injection study on the thoracodorsal artery. Acta Anat. 1988;131:97-102.
18. BRICOUT N, SERVANT JM. Fermature de la zone de prélèvement dun lambeau de grand dorsal. Ann. Chir. Plast. Esthet. 1987;2:228-7.
19. FEARON JA, CUADROS CL, MAY JW. Flap failure after microsurgical free tissue transfer: The fate of a second attempt. Plast. Reconstr. Surg. 1990;86:746-51.
20. TESTUT L. Traité d'anatomie humaine. 7ème. ed. Paris, Octave Doin, 1921. 3 vols.
21. JONES NF, JOHNSON JT, SHESTAK KC, MYERS EN, SWARTZ WM. Microsurgical reconstruction of the head and neck: interdisciplinary collaboration between head and neck surgeons and plastic surgeons in 305 cases. Ann. Plast. Surg. 1996;36:37-43.
22. CARIOU JL. Transferts tissulaires libres en chirurgie plastique (urgences exclues) a propos d'une experience de dix ans. Ann. Chir. Plast. Esthet. 1992;37:460-1. [Comentaire].
I - Head of the Reconstructive Plastic Surgery Service of the Federal University of (UFAL) - Brazil.
II - Head of the Plastic Surgery Service of Saint-Louis Hospital - Paris - France.
III - Head of the Plastic Surgery Service of UNIFESP [Federal University of São Paulo]- São Paulo - Brazil.
IV - Assistant of the Plastic Surgery Service of Saint-Louis Hospital - Paris - France.
V - Assistant of the Plastic Surgery Service of UNIFESP [Federal University of São Paulo]- São Paulo - Brazil.
VI - Medical Student of the Federal University of Alagoas - Brazil.
Address for correspondence:
Centro de Saúde (CSAU)
Campus Universit. A. C. Simões BR 101 Norte Km 14
57081-000 - Maceió - AL Brazil
Phone/Fax: (55 82) 221-5081
Dept. Clínica Cirúrgica - Disciplina de Cirurgia Plástica Reconstrutora - Prof. Dr. Fernando Antônio Gomes de Andrade


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter