

Articles - Year 2000 - Volume 15 -
Autologous Graft of Cultivated Epidermis
Enxerto Autólogo de Epiderme Cultivada
ABSTRACT
With the modern techniques of tissue culture, it is possible to obtain from a small fragment of skin, laminae of cultivated keratinocytes to be used for covering raw areas. This cellular population can be rapidly expanded, reachinq, at the end of 30 days, a dimension hundreds of times bigger than the original biopsy. The authors analyze the method used for the keratinocytes culture, the difficulties found, the present applications and future possibilities.
Keywords: Skin culture; keratinocytes; coverage of raw areas
RESUMO
As técnicas atuais de cultura de tecidos permitem obter, a partir de um pequeno fragmento de pele, lâminas de queratinócitos cultivados para serem utilizados na cobertura de áreas cruentas. Essa população celular pode ser rapidamente expandida, atingindo, ao final de 30 dias, dimensão centenas de vezes maior do que a da biópsia inicial. Os autores analisam o método utilizado para a cultura de queratinócitos, as dificuldades encontradas, as aplicações atuais e as possibilidades futuras.
Palavras-chave: Cultura de pele; queratinócitos; cobertura de áreas cruentas
Loss of skin is a problem that torments the everyday of Plastic Surgery. We are constantly planning and executing procedures aiming at covering raw areas, not only those of traumatic origin, such as those produced by us after the removal of lesions, but also the donor areas of grafts and flaps. The ideal substitute for skin, which must be good, inexpensive and highly available, has not yet been found. The culture of cells seems to be a promising route since the technological development of this area highlights it as an efficient, relatively fast and economically viable procedure.
Obtaining human keratinocytes, in the form of a confluent layer, capable of being used as a graft, has been first described by Green(1), in 1979. Based on this pioneer work, improvements on the technigue have been introduced, as well as its several applications(2, 3 and 4).
Together with the Cell Bank of Rio de Janeiro - PABCAM, located at the Hospital Universitário Clementino Fraga Filho, a clinical-laboratory study was initiated in 1997, in order to systemize the culture, in vitro, of human epidermis and make its practical applications possible. This technigue is used in important medical centers worldwide, but is not common yet in Brazil.
From cutaneous biopsy, we can obtain fragments of cultivated autologous epidermis, which allow the covering of raw areas of several extensions and etiologies, such as: burns, chronic ulcers, epidermolysis bullosa, donor areas of cutaneous grafts or areas resulting from recession of giant nevi, etc. Another possible indication is the treatment of some cases of vitiligo, due to the presence of melanocytes in the culture.
MATERIAL AND METHOD
CLINICAL PRASE
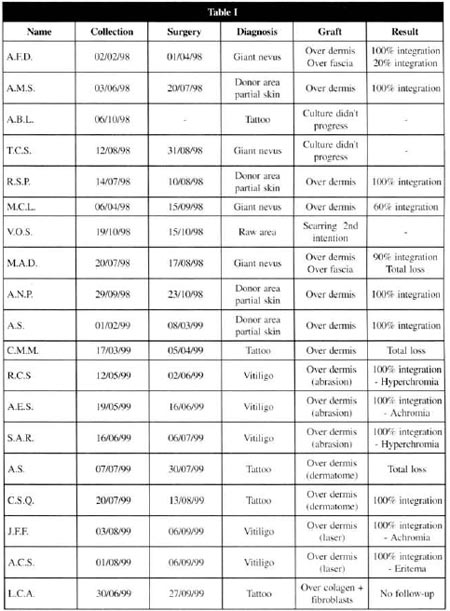
In the period between April, 1998 to October, 1999, 19 patients with giant nevi (4), tattoos (5), vitiligo (5), or who would be submitted to the removal of partial skin grafts for the covering of complex wounds (5) were selected. Ten patients were females and 9 were males. The ages ranged from 8 months to 72 years.
At the first visit, the need for immediate cutaneous coverage or for the coverage after the treatment of the basic lesion was evaluated. In accordance with the Norms for Research in Human Beings, the procedures were explained to the patients and they were asked whether they accepted them. Once they had agreed, a medical file was created at the Institution, followed by anamnesis and physical examination, for the beginning of treatment.
The biopsy was performed under local anesthesia, in an outpatient basis (15 patients), or during the surgery of another procedure, under general anesthesia (4 patients), obtaining fragments of about 2 cm2.
This material was taken to the Cell Bank for cultivation, for an average period of 30 days, during which the complementary examinations and the clinical preparation of the patients were performed.
LABORATORY PHASE
The technigue described by O'Connor(16) in 1981 was used with a few changes. The cutaneous fragments were transported to the laboratory in Eagle's culture medium, modified by Dulbecco (DMEM) (Sigma Chemical Co., St. Louis, MO, USA), with fetal bovine serum at 10% (FBS) (Cultilab,Campinas, SP, Brazil) and 1 µg/mL amphotericin B (Eurofarma Laboratórios Ltda., São Paulo, SP, Brazil), under refrigeration. The largest possible amount of skin was removed, and the fragmems containing the epidermis were fragmented with a scalpel. This latter portion was then submitted to enzymatic dissociation, with 0,3% trypsin (Sigma) in a balanced salt solution calcium- and magnesium-free (BSS-CMF), during 18 to 20 hours, at 4°C, followed by incubation in a new solution, from 30 to 60 minutes at 37°C, with orbital jar. The epidermal cells, now apart from each other, were plated in culture bottles (Nunc, Roskilde, Denmark), 6x106 cells/75 cm2, containing 3T3 (embryonal cells from mice) as a feeding layer. The 3T3, obtained from the Cell Bank of Rio de Janeiro, was previously cultivated until it reached confluence, and then it was treated with 10,ug/mL Mitomicyn C (Sigma), so it would not proliferate anymore (Figs. 1 and 2).
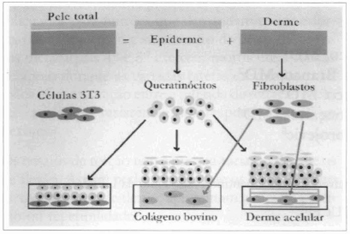
Fig. 1 - Scheme of the 3 types of procedures used: using the dispase enzyme, the epidermis and the corium are dissociated, resulting in keratinocytes, from the first, and fibroblasts, from the second. On the left, the scheme shows keratinocytes sowed over a 3T3 cells feeding layer. In the center, fibroblasts are sowed over collagen gel, forming a new marrix over which keratinocytes are sowed. On rhe right, acellular corium is populated by fibroblasts and supports the culture of keratinocytes.
Fig. 2 - Epidermal cells are plated in bottles containing embryonal cells from mice (3T3) as the feeding layer. The culture's evolution is daily monitored by means of a microscope, until a confluence of the keratinocytes is observed, which occurs after 20 to 40 days.
The cultures were kept for 20 to 40 days in medium for keratinocytes: minimum essential medium, D-valine modification, supplemented with 5 mg/L insulin, 4mM L-glutamine, 0,4 mg/mL hydrocortisone, 10-10 M cholera toxin, 5 mg/L transferrin, 2xlO-9 M T3, 10ng/mL epidermal growth factor (all from Sigma) and fetal bovine serum at 20% (Cultilab), at 37°C and CO2 at 5%. Cellular growth was monitored by means of an inverted microscope with phase contrast. The culture medium was changed every 2 or 3 days. Cellular confluence was reached in a period of 10 to 20 days, depending on the patient's age (Figs. 3 and 4). After 4 to 7 days in confluence, the epidermal layer was removed from the bottle, using 1.2 U/mL dispase in DMEM (Sigma), for 60 minutes at 37°C. This layer was then washed with a balanced salt solution (BSS), fixed on a gauze soaked with petrolatum (Adapatic®), using surgical clips (Ligaclip - both from Johnson & Johnson Medical INC., São Paulo, Brazil), transported to the surgery center on Petri dishes, which were refrigerated, and used for grafting (Figs. 5 and 6). The period between cell removal and grafting was of 4 hours (Fig.7).
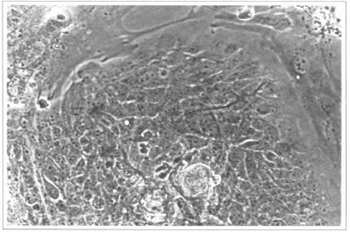
Fig. 3 - Epidermal culture (9th day) - colonies of epidermal cells can be observed; they are surrounded by fibroblasts from the feeding layer. 200 x inverted microscope.
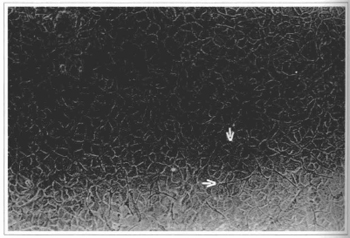
Fig. 4 - Epidermal culture (21stday) - cellular confluence and the presence of melanocytes can be observed. Culture ready to be removed, 100x inverted microscope.
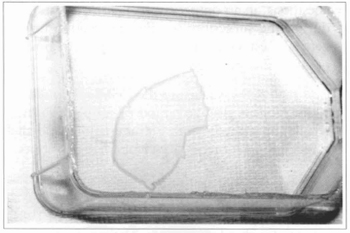
Fig. 5 - After 4 to 7 days in confluence, the cultivated epidemis is enzymatically removed from the bottle, using dispase.
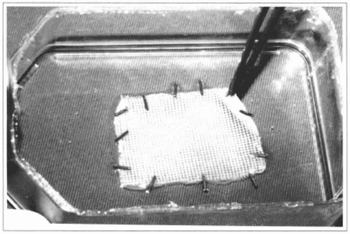
Fig. 6 - The cultivated epidermis lamina is framed over a gauze soaked with petrolatum, using surgical clips.
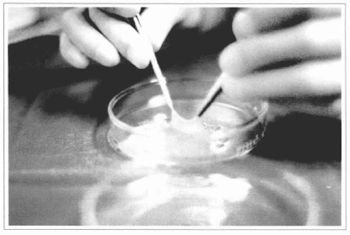
Fig. 7 - The gauze + epidermis sets are taken to the surgery center, observing the proper asepsis and refrigeration. In this figure, the epidermis has been elevated from its gauze bed to show its thickness.
SURGICAL PHASE
The grafts were applied directly on the main raw area in 11 cases, or as a complement to the method used for covering this area in 6 cases (Figs. 8 and 9). The receptor bed was prepared according to the basic disease: with scalpel (4 cases), deepithelization with CO2 laser (2 cases), abrasion with water-resistant sandpaper (3 cases), deepithelization with electric dermatome (8 cases).
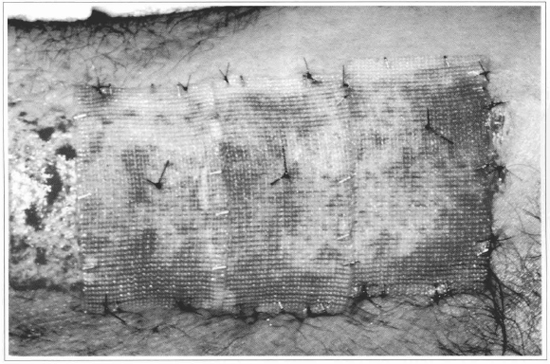
Fig. 8 - The gauze + epidermis sets are fixed over the raw area, and the gauze is used to support the fixation points. In this case, a non-grafted strip was left, so as to allow the comparison with the integration of the cultivated epidermis.

Fig. 9 - After 10 days, complete epithelization of the treated area can be observed, noticing that the grafted part with the cultivated epidermis presents a thicker covering than the strip which epithelized without grafting. The limit between the two parts is marked by two points.
Due to their reduced thickness, the cultivated skin fragments were framed to gauze with petrolatum, which served as an anchor to the stitches, 5-0 mononylon. All the set was covered with vaseline gauze and tie-over dressing. The dressing was removed after 5 to 7 days for evaluation, keeping the area covered with soft gauze for at least 14 days.
RESULTS
From the 19 patients initially selected, 1 abandoned the treatment, 1 presented healing by second intention in the area to be treated and, in 2 of them, the culture did not evolve, and another repair technique was applied at the laboratorial phase. Thus, a total of 15 patients was reached, with 16 treated areas.
At the postoperative period, it was observed that the 9 patients presented a complete integration of the grafts, 3 had an integration varying from 60 to 90 %, and 2 of them did no present any integration (Table I) .
DISCUSSION
The culture of cells is hindered, at first, by the elevated costs of the equipment involved and of the staff training. It is further hindered by technical secrets and patent problems, since major laboratories have been acting on the industrial production of cutaneous substitutes.
Therefore, even though the basic techniques can be found in a number of publications, the small details of the execution, which are vital to the complete performance of the procedures, need to be rediscovered, individually, by different researchers.
The constant lack of funds and the scarcity of specialized technicians available add up to the difficulties. All this, together with the attempts, by other institutions, to take the few skilled professionals we have, by means of attractive financial proposals, makes up the scenario in which university research is developed in our country.
On the other hand, however sophisticated, research on cell culture is perfectly practicable and of current interest. Ir is also surrounded by a touch of magic, since the ability to multiply, in laboratories, human beings, wholly or in part, is still perceived as a novelty, even after some decades of observation. And, most importantly, these are not routine procedures, which justifies new and varied research.
Once we were not familiar with the technique, we chose cases in which the failure of the grafting would not bring about any inconvenience other than the small scar on the biopsy area, that is:
1) Donor areas of partial skin grafts, where the epithelization would normally occur, even without the graft of cultivated skin.
2) Giant nevi, which usual treatment consists of multiple ressections. If the graft did not integrate, we would proceed to the previously planned ressection.
3) Vitiligo - the receptor areas were prepared by means of dermabrasion with sand paper or laser. If there was no integration of the cultivated skin, there would be spontaneous epithelization.
4) Tattoos - the tattooed area could be abraded or deepithelized with dermatome or scalpel. The layer of cultivated skin would accelerate the epithelization, and also contribute to disguise residual pigments. If there was no integration, epithelization would occur spontaneously.
A preliminary reading of several published articles misled us of the possible applications of the cultivated epidermis. O'Connor and colleagues(6)(1981) published the first clinical application of cultivated keratinocytes, used in two patients with extensive third degree burns. They tested the grafting on three types of receptor bed: recent granulation, chronic granulation and recently-debrided fascia. They refer to satisfactory integration of the cultivated epidermis in both patients and in the three types of raw areas. However, they also reported that "both patients were extensively grafted with expanded autologous grafts" and that "after 3 to 4 weeks, the cultivated epidermis reached the surrounding expanded skin", which leads to the conclusion that the grafts were placed on the orifices of the expanded skin or very close to them. It is impossible to guarantee if the resulting epidermis was the cultivated one or was a result of the growth of the expanded skin borders.
Other authors presented their experience with cultivated epidermis on burnt patients, but with heterogeneous results(7, 8 and 9).
Gallico e colleagues(10) (1989) used cultivated epidermis to cover raw arcas resulting from the removal of giant nevi in 8 children. The ressections reached down to the fascia, over which the grafes were placed. They refer to an average integration of 68% with a maximum of 84% in one case.
Our experience does not confirm these results. We tried to place cultivated epidermis segments directly over raw areas of several types; however, we concluded that the epidermis does not integrate with irregular surfaces and where there is no corium. We also considered equaliy doubtful the possibility of deriving any benefits from the placement of cultivated epidermis over burnt areas. This fact was confirmed when we tried to graft the epidermis over muscular fascias, in the patients who had giant nevi, after the ressection of the impaired tissues. There was no integration and, positively, the surface of the fascia is a better receptor area than the burnt surfaces (Figs. 10,11,12 and 13).
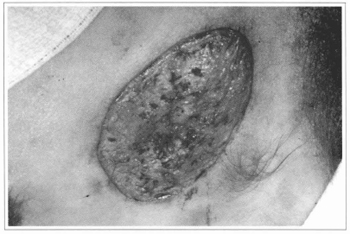
Fig.10 - Ressection of the nevus preserving the dermal bed, over
which the cultivated epidermis will be grafted.
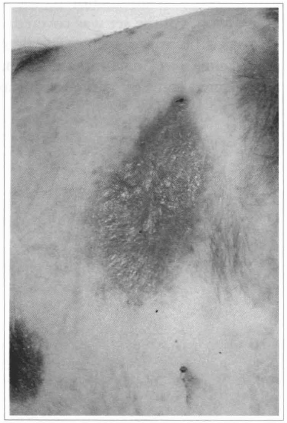
Fig. 11 - Ten days after the cultivated epidermis grafting, complete epithelization can be observed.
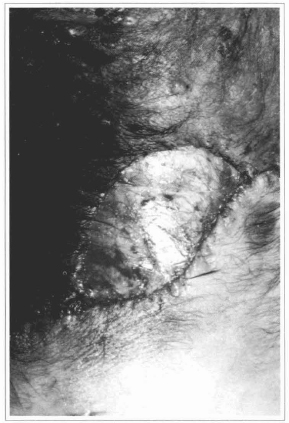
Fig. 12 - Ressection of the nevus with exposition of the deep fascia, over which the epidermis will be grafted.
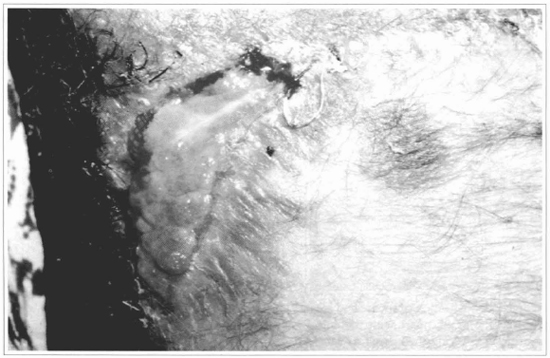
Fig. 13 - After 15 days, a complete loss of me graft can be observed.
We believe that the use of isolated epidermis should be limited to raw areas which have corium.
The question as to what is the advantage of using cultivated epidermis to cover areas that will epithelize spontaneously, such as donor areas of grafts, can be answered by the patients themselves. Every plastic surgeon knows that raw areas resulting from the removal of split-thickness grafts are extremely painful and adherent to dressings, thus making their removal more difficult; besides, such areas produce serum-hematic secretion, which produces local bad smell and is a culture medium for bacteria. The discomfort on the donor area is always worse than that on the area of the main disease which impelled the graft removal. This lasts for at least two weeks, if there are no complications.
The use of cultivated skin totally eliminated the pain and the secretions, producing complete epithelization of the wounds in less than 7 days. A strip of raw area, over which no graft was placed, was left in all patients. The external checking of the dressing always highlighted moistening on that area and the patient explained that the pain was limited to it.
Another attempt was made with patients suffering from vitiligo. Several articles recommend this procedure, due to the fact that, among the keratinocytes, some melanocytes are cultivated (11,12) (Fig. 14). In our 5 patients, we prepared the recepient areas with dermabrasion(3) or laser(2). The graft integration was complete in all cases, after around 7 days, and the patients were instructed to follow the usual dermatological therapies for the disease. However, after 6 months of follow-up, we concluded that the results are poor ar insufficient, since the pigmentation either does not occur or is irregular, both in tint and distribution. Therefore, we consider that, for such patients, the used techniques still have to be improved (Figs. 15, 16 and 17).
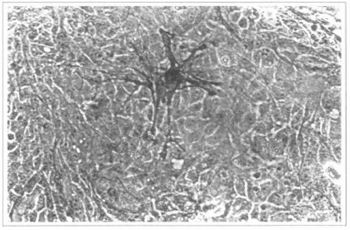
Fig. 14 - Epidermal culture (9th day). The present melanocyte is highlighted. 200x inverted microscope.
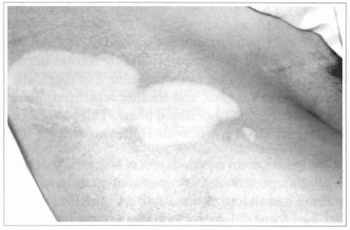
Fig. 15 - Achromic area in parient suffering from vitiligo. An abrasion with water-resistant sandpaper was performed and cultivated epidermis was grafted.
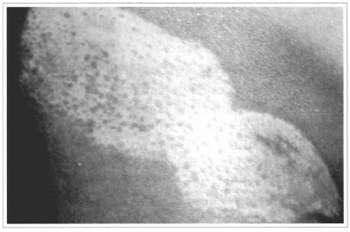
Fig. 16 - Local appearance, 4 months after the grafting, observing a subtle and sparse pigmemation.
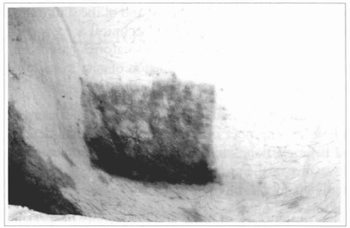
Fig. 17 - Another patient suffering from vitiligo, in whom cultivated epidermis was grafted, dose to the pubic hairs, over abraded area. Appearance after 5 months, observing hyperchromia and irregular distribution of the pigments.
The experience acquired with keratinocytes culture indicates that there is the need to develop artificial or biogenous supports to substitute the skin. At the moment, we are investigating two kinds of dermal substitution: bovine collagen and human acellular corium, both containing human fibroblasts (Figs. 18 and 19). Such substitutes would serve both for temporary and permanent coverage. In the first case, they would prepare the raw area for subsequent grafting, minimizing the pain and hydroelectrolytic losses. The definite coverage of the raw area could also be attained, by means of the association of skin substitutes and epidermal cells cultivated in their surface or subsequently applied. Both possibilities seem to be promising and will motivate further research.
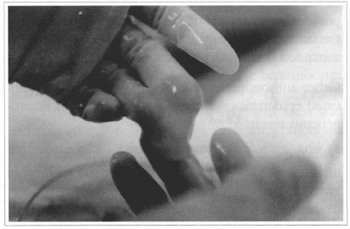
Fig. 18 - Bovine collagen preparation with fibroblasts, to be used as a dermal substitute.
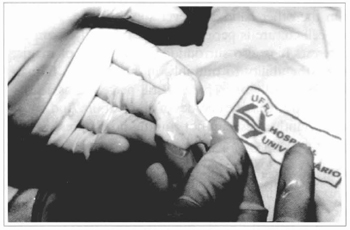
Fig. 19 - Acellular corium preparation.
CONCLUSIONS
a) The culture of keratinocytes is a viable technique, capable of producing large tissue segments from small biopsies, in short periods of time.
b) The integration of the cultivated epithelial laminae occurs perfectly on dermal surfaces, promoting a faster epithelization and eliminating the pain and the secretions from the wound.
c) The cultivated epidermis does not integrate over raw areas without corium.
d) The use of cultivated epidermis on vitiligo areas did not result in homogeneous pigmentation.
REFERENCES
1. GREEN H et al. Growth of cultured human epidermal cells into multiple ephitelia suitable for grafting. Proc. Natl. Acad. Sci. USA. 1979;76:5665-5668.
2. TSAI C et al. Clinical results of cultured epithelial cell grafting in the oral and maxillofacial region. J. Cranio-Maxillofacial Surg. 1997;25:4-8.
3. FAHMY FS et al. Skin graft storage and keratinocyte viability. British Journal of Plastic Surgery. 1993;46:292-295.
4. FALABELLA R, ESCOBAR C, BORRERO I. Treatment of refractory and stable vitiligo by transplantation of in vitro cultured epidermal autografts bearing melanocytes. J. Am. Acad. Dermatol. 1992;26:230-236.
5. GOBET R et al. Efficacy of cultured epithelial autografts in pediatric burns and reconstructive surgery. Surgery. 1997;121:655-661.
6. O'CONNOR NE, MULLIKEN JB, BANKS-SCHLEGEL S, KEHINDE O, GREEN H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet, 1981;1:75-78.
7. COLEMAN JIII, SIWY B.Cultured epidermal autografts: a life saving and skin saving technique in children. J. Pediatr. Surg. 1992;2:210-215.
8. McAREE K, KLEIN R, BOECKMAN C. The use of cultured ephitelial autografts in the wound care of severely burned patients. J. Pediatr. Surg. 1993;28:166-168.
9. BLIGHT A, MOUNTFORD E, CHESHIRE I, CLANCY J, LEVICK P. Treatment of full skin thickness burn injury using cultured ephitelial grafts. Burns. 1991;17:495-498.
10. GALLICO GGIII, O'CONNOR NE, COMPTON CC. Cultured epidermal autografts for giant congenital naevi. Plast. Reconstr. Surg. 1989;84:1-9.
11. OLSSON MJ, JUHLIN L. Transplantation of melanocytes in vitiligo. Br. J. Dermatol. 1995;132:587-591.
12. KAUFMANN R, GREINER D, KIPPENBERGER S, BERND A. Grafting of in vitro cultured melanocytes onto Laser-ablated lesions in vitiligo. Acta Derm. Venereol. 1998;78:136-138.
I - Head of the Plastic Surgery Service of the Hospital Universitário Clementino Fraga Filho da UFRJ.
II - Biotechnologist of the Cell Bank of Rio de Janeiro (HUCFF).
III - Doctorate student - HUCFF-UFRJ.
IV - Doctorate student - HUCFF-UFRJ.
V - Director ofthe Cell Bank of Rio de Janeiro - HUCFF.
Address for correspondence:
Talita Franco, MD
Praia de Botafogo, 528 apto 1402-B
22250-040 - Rio de Janeiro - RJ Brazil
Fax: (55 21) 542-7119
e-mail: talita@openlink..com.br


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter