

Original Article - Year 2020 - Volume 35 -
Histological comparison between irradiated and non-irradiated breasts in breast reconstruction
Comparação histológica entre mamas irradiadas e não irradiadas em reconstrução mamária
ABSTRACT
Introduction: The treatment of breast cancer includes not only curative therapies but also breast reconstruction. Radiotherapy, an adjuvant strategy, provides favorable outcomes by reducing the rate of recurrence of the disease. This study aimed to compare histological differences between irradiated and non-irradiated breasts in the same patient.
Methods: This is a prospective cohort study of patients undergoing breast reconstruction with prosthesis or expander under pectoralis major muscle flap that compared histological skin patterns, subcutaneous cell tissue, pectoralis major muscle, and implant capsule of irradiated and non-irradiated breasts in paired samples of the same patient. All patients included in this study were irradiated in only one breast. The results of the anatomopathological analysis were compared to clinical findings and intraoperative macroscopic aspects.
Results: The study included a total of 7 patients with a mean age of 52.15 years. The main histological findings in the skin and subcutaneous cellular tissue of the irradiated breast were as follows: epidermal hyperplasia, flattening of the papillary layer, atrophy of the skin appendages, vascular congestion in fatty tissue, high density of skin collagen fibers, hyalinization, and reduction of elastic fibers in the deep dermis and unidirectional alignment of collagen fibers. The main histological findings for the capsule and pectoralis major muscle in the irradiated breast were as follows: lower density of elastic fibrosis, perivascular fibrosis, synovial metaplasia, skeletal muscle sequestration at the interface with the capsule, capsular hyalinization, and capsular fribrosclerosis.
Conclusion: We found common histological changes in irradiated breasts in most patients. These findings are compatible with the clinical and macroscopic changes observed. This study presents itself as a pilot for the development of further studies investigating the physiopathological mechanisms of the described histological changes.
Keywords: Breast; Breast neoplasms; Radiotherapy; Adjuvant radiotherapy; Prostheses and implants.
RESUMO
Introdução: O tratamento do câncer de mama inclui, além de terapias curativas, a reconstrução mamária. Entre as estratégias adjuvantes, a radioterapia fornece desfechos favoráveis em termos de redução da taxa de recorrência da doença. Esse estudo tem como objetivo comparar as diferenças histológicas entre mamas irradiadas e não irradiadas em um mesmo paciente.
Métodos: Estudo prospectivo de coorte em pacientes submetidos à reconstrução mamária com prótese ou expansor sob retalho muscular de peitoral maior, comparando os padrões histológicos de pele, tecido celular subcutâneo, músculo peitoral maior e cápsula do implante, de mamas irradiadas e não irradiadas em amostras pareadas de um mesmo paciente. Todos os pacientes deveriam receber irradiação em apenas uma das mamas. A análise anatomopatológica foi comparada aos achados clínicos e aos aspectos macroscópicos do transoperatório.
Resultados: O trabalho contou com um total de 7 pacientes, sendo a idade média de 52,15 anos. Os principais achados histológicos em pele e tecido celular subcutâneo da mama irradiada foram: hiperplasia epidérmica, achatamento da camada papilar, atrofia dos apêndices dérmicos, congestão vascular no tecido gorduroso, alta densidade das fibras de colágeno dérmico, hialinização das paredes vasculares, redução das fibras elásticas na derme profunda e alinhamento unidirecional das fibras de colágeno. Os principais achados histológicos de cápsula e músculo peitoral maior na mama irradiada foram: menor densidade de fibras elásticas, fibrose perivascular, metaplasia sinovial, sequestro de músculo esquelético na interface com a cápsula, hialinização capsular e fibroesclerose capsular.
Conclusão: Encontramos alterações histológicas comuns nas mamas irradiadas em boa parte das pacientes, achados esses que são compatíveis com as alterações clínicas e macroscópicas observadas. Esse estudo apresenta-se como um piloto para o desenvolvimento de novos estudos que pesquisem os mecanismos fisiopatológicos relacionados às alterações histológicas descritas.
Palavras-chave: Mama; Neoplasias da mama; Radioterapia; Radioterapia adjuvante; Próteses e implantes
INTRODUCTION
Since the 1990s, the treatment of breast cancer has included not only curative therapies but also breast reconstruction, which has increased patients’ interest in getting protected from cancer and obtaining better aesthetic results. Many studies have reported that breast reconstruction has no negative impact on cancer safety, reassuring patients and motivating them to perform the procedure1,2.
Studies show that radiotherapy, an adjuvant strategy, provides favorable outcomes by reducing the rate of recurrence of the disease, thus increasing the number of indications. The guidelines for radiotherapy began to include more diverse indication criteria, further expanding the use of this therapeutic modality3,4.
Immediate reconstruction is indicated because it can significantly improve the quality of life of a woman, helping in her body image satisfaction and psychosocial well-being, compared to delayed reconstruction5.
Breast reconstruction using implants is a relatively simple procedure, with short surgical time and rapid postoperative recovery. It also provides excellent aesthetic results. Its advantages compared to autologous flap reconstruction include smaller procedures with good results, without the need to transpose skin islands from other regions of the body to the breast6,7. Given these facts, it is currently one of the most popular surgical methods. The increasing indication of breast reconstruction with implants, combined with the full application of radiotherapy, have made it more difficult to understand and avoid potential complications associated with the interaction between radiation and implant. Capsular contracture, infection, and poor positioning of the breast implant were indicated as the main complications. Although capsular contracture is the most important complication, being the most common cause of reoperation,3,8,9 its pathogenesis induced by radiation is still unknown10.
Some of the skin changes after irradiation have been investigated, and early effects (up to 90 days after the onset of radiation) include dehydration, pigmentation changes, loss of skin appendages, erythema, and desquamation. Delayed histological changes (after 90 days) include atrophy or hyperplasia of the epidermis, hypocellular fibrosis of the dermis, sclerotic vascular changes, and absence of pilosebaceous units (appendages). However, it is still unclear whether these changes are associated with the difficulties and complications of breast reconstruction with expander/implant11,12. The susceptibility of the skin to changes after irradiation can be determined genetically. This concept is reinforced by individual differences in changes caused by radiation and developed complications13,14,15.
This study aimed to describe and compare histological differences between skin, subcutaneous cell tissue, pectoralis major muscle, and capsule of irradiated and non-irradiated breast implant in the same patient and to guide further studies to analyze possible methods of prophylaxis and treatment of complications. Currently, there are no studies that address such comparisons on all tissue layers.
METHODS
Study design
Patients and tissue collection
This was a prospective cohort study of patients who underwent breast reconstruction with prosthesis or expander under pectoral major muscle flap, between January and August 2019, at the Daher Lago Sul Hospital, Brasília (DF). The histological patterns of skin, subcutaneous cellular tissue, pectoralis major muscle, and capsule of irradiated and non-irradiated breast implant were compared in paired samples of the same patient. All patients signed an informed consent form authorizing and agreeing to undergo surgical procedures and anatomopathological examinations and to record them for scientific purposes. The study was submitted to Plataforma Brasil, and the manuscript was validated by the Research Ethics Committee of the Health Sciences Teaching and Research Foundation/FEPECS/SES/DF, whose CAAE number is 15942719.6.0000.5553.
Surgeries were performed at the Daher Lago Sul Hospital–DF and the Brasília Hospital– DF. All selected patients had a history of previous radiotherapy in another institution, following the current reference scheme (50 Gy), which consists of 25 radiotherapy sessions for five weeks plus an additional dose on the tumor bed. Patients older than 18 years undergoing breast reconstruction for treatment of capsular contracture or other complications of adjuvant radiotherapy or contralateral symmetrization were included. Patients that were included in this study received irradiation in only one breast.
Biopsy tissues were collected during breast reconstruction. Samples of skin and subcutaneous cell tissue with length, width, and depth ranging between 0.5 and 1.0 cm were taken bilaterally from the submammary sulcus.
Samples of capsular tissue and pectoralis major muscle with dimensions 1.0 × 1.0 cm were taken from the site of greatest cicatricial retraction in the irradiated breast and in the inferomedial portion near the submammary sulcus of the non-radiated breast.
Anatomopathological analysis was performed, and the results were compared to the clinical findings of physical examination and the transoperative macroscopic aspects such as color, elasticity, vascularization, healing, and tissue sensitivity, to establish correlations between the clinical/histological variables and radiation.
Histological approach
The histological processing of tissue specimens was performed according to the method described by Huanget et al., in 201616. Briefly, tissue specimens were fixed in buffered formaldehyde, embedded in paraffin, and stained by hematoxylin-eosin, Masson’s trichrome, and Voerhoff elastic fibers for light microscopy. The histological evaluation was performed by a single pathologist in the Diagnose laboratory, Brasília (DF).
Quantitative and qualitative aspects of the epidermis were evaluated. Panniculus and implant capsule in the dermis were quantitatively and qualitatively evaluated for collagen, cellularity, inflammation, and vascularization.
RESULTS
The data from 7 patients, all female, with a mean age of 52.15 years (ranging from 34 to 68 years) were analyzed. They received 25 sessions of conventional radiotherapy in one breast, with a total dose of 50 Gy. The mean time between the last radiotherapy session and breast reconstruction was 54.14 months (ranging from 7 to 204 months). One of the postoperative complications of the first surgery was capsular contracture, which affected all patients and led to the second surgical period. One patient had suture dehiscence on both breasts, which was resolved with local bandages. None of them had infections.
While skin and subcutaneous cell tissue biopsies were collected in all patients, capsule and pectoralis major muscle biopsies were also collected, since the breast contralateral to radiotherapy did not receive the implant. The main clinical and macroscopic changes during the surgical procedure were as follows: dry skin (100%), loss of skin elasticity (85.71%), hypovascularized fat (100%), capsular contracture (100%), capsular thickening (85.71%), muscle hypotrophy (100%), hypovascularized muscle (85.71%), and skin dyschromia (42.85%), as shown in Table 1.
| Case | Time between last radiation and surgery |
Irradia ted side |
Clinical and macroscopic changes of irradiated breast | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DS | LSE | HF | CC | CT | MH | HVM | SD | ||||
| 1 | 57 | 108 months | Left | + | + | + | + | + | + | +/- | - |
| 2 | 68 | 204 months | Right | + | + | + | + | +/- | + | + | + |
| 3 | 60 | 24 months | Left | + | + | + | + | + | +/- | + | + |
| 4 | 34 | 18 months | Right | + | + | + | + | + | +/- | + | - |
| 5 | 42 | 10 months | Left | + | - | +/- | + | +/- | +/- | - | - |
| 6 | 40 | 7 months | Right | +/- | +/- | + | + | + | + | + | +/- |
| 7 | 64 | 8 months | Right | + | + | +/- | + | - | + | + | - |
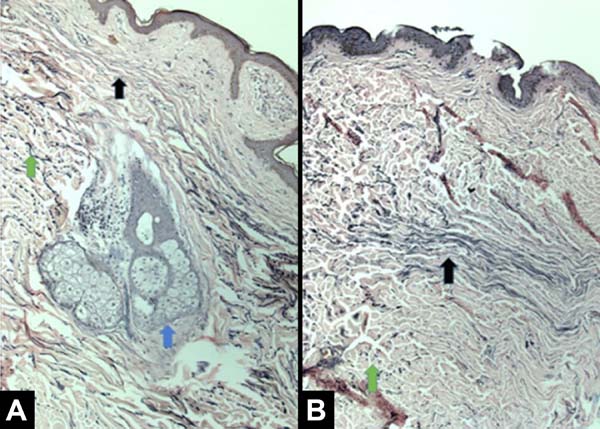
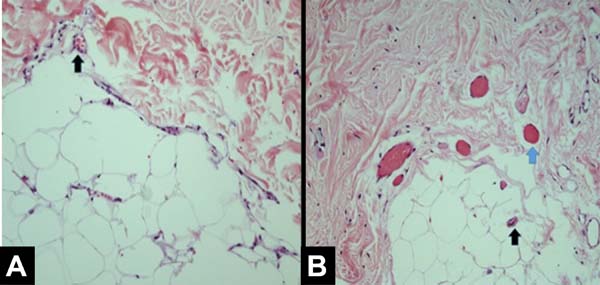
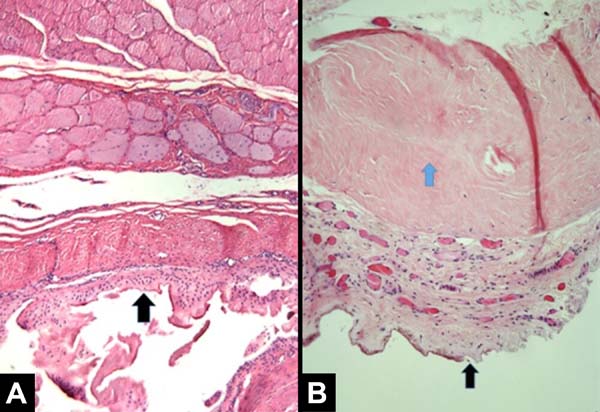
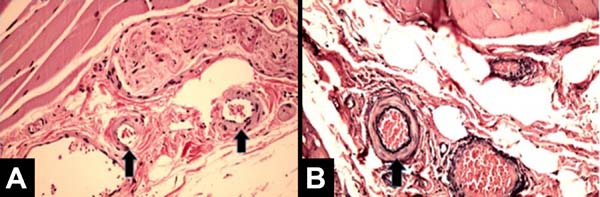
The main histological findings in the skin and subcutaneous cellular tissue in the irradiated breast were as follows: epidermal hyperplasia (71.42%), flattening of the papillary layer (85.71%), atrophy of the skin appendages (100%), vascular congestion in fatty tissue (71.42%), high density of skin collagen fibers (100%), hyalinization of vascular walls (85.71%), reduction of elastic fibers in the deep dermis (85.71%), and unidirectional alignment of collagen fibers (100%), as shown in Table 2. These findings were observed in the samples of irradiated skin with considerable differences.
| Case | Age | Time between last radiation and surgery |
Side irradiate |
Irradiated breast | Non-irradiated breast | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EH | FPL | ASA | VC | HDCF | HVW | RFEDP AUFC |
EH | FPL | ASA | VC HDCF |
HVW | RFEDP AUFC |
||||
| 1 | 57 | 108 months | E | + | + | + | + | + | + | + + | - | - | - | - - | - | - + |
| 2 | 68 | 204 months | D | +/- | + | + | + | +/- | + | + + | - | - | - | - + | - | +/- - |
| 3 | 60 | 24 months | E | + | +/- | + | + | + | + | + + | - | + | - | - - | - | - - |
| 4 | 34 | 18 months | D | + | + | + | + | + | +/- | + + | - | +/- | - | - - | +/- | - - |
| 5 | 42 | 10 months | E | - | + | +/- | +/- | + | +/- | +/- + | - | - | - | +/- - | - | - - |
| 6 | 40 | 7 months | D | + | - | + | - | +/- | +/- | - +/- | - | - | - | - +/- | - | - - |
| 7 | 64 | 8 months | D | - | + | + | - | +/- | - | +/- +/- | - | - | - | - - | - | - - |
EH: Epidermal hyperplasia; FPL: Flattening of the papillary layer; ASA: Atrophy of the skin appendages; VC: Subcutaneous vascular congestion; HDCF: High density of collagen fibers; HVW: Hyalinization of the vascular wall; REFDD: Reduction of elastic fibers in the deep dermis; UACF: Unidirectional alignment of collagen fibers; * skin and subcutaneous cell tissue analysis; "- " (not found); "+/- " (found moderately); "+" (found).
The main histological findings of capsule and pectoralis major muscle in the irradiated breast were as follows: lower density of elastic fibers (80%), perivascular fibrosis (100%), synovial metaplasia (100%), skeletal muscle sequestration at the interface with the capsule (80%), capsular hyalinization (80%), and capsular fibrosclerosis (100%), as shown in Table 3.
| Case | Age | Time between last radiation and operation |
Degree of capsular contractur |
Irradiated side |
Irradiated breast | Non-irradiated breast | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDEF | EF | SM | SMS | CH | CF | LDEF | EF | SM | SMS | CF | CF | |||||
| 1 | 57 | 108 months | Baker 3 | E | + | + | + | +/- | + | + | - | +/- | - | - | - | - |
| 2 | 60 | 24 months | Baker 2 | E | +/- | + | + | + | + | + | - | - | - | +/- | - | - |
| 3 | 34 | 18 months | Baker 3 | D | + | +/- | + | + | + | + | - | - | - | - | - | - |
| 4 | 42 | 10 months | Baker 2 | E | + | + | + | +/- | +/- | +/- | - | - | - | - | - | - |
| 5 | 40 | 7 months | Baker 2 | D | + | - | + | - | - | + | - | - | - | - | - | - |
All patients had similar anatomical findings, which were consistent with clinical findings. It should be mentioned that cases 5 and 7 had more discreet anatomopathological findings, occasionally having a mixed pattern of presentation with areas containing normal tissue in the irradiated breast. Figures 1, 2, 3, and 4 show the main histological differences between irradiated and non-irradiated breasts.
Clinical cases
Case 1
A 36-year-old patient, without comorbidities, diagnosed with invasive ductal carcinoma in the right breast, undergoing neoadjuvant chemotherapy + bilateral mastectomy without preservation of nipple-areola-complex, with right axillary dissection + immediate reconstruction with retromuscular remote valve expanders, in February 2016, without complications. Expansion was performed with physiological solution until March 2016 and adjuvant radiotherapy was performed in the right breast (last session in June 2016). Submitted to second surgical period in February 2017 for replacement of expander by silicone implants + bilateral reconstruction of nipple-areola-complex + liposuction of axillary extensions without complications. Due to the development of intense radiodermatitis not responsive to clinical treatments, she underwent microsurgical reconstruction of the right breast with transverse flap of the rectus abdominis muscle in June 2019, as shown in Figure 5.
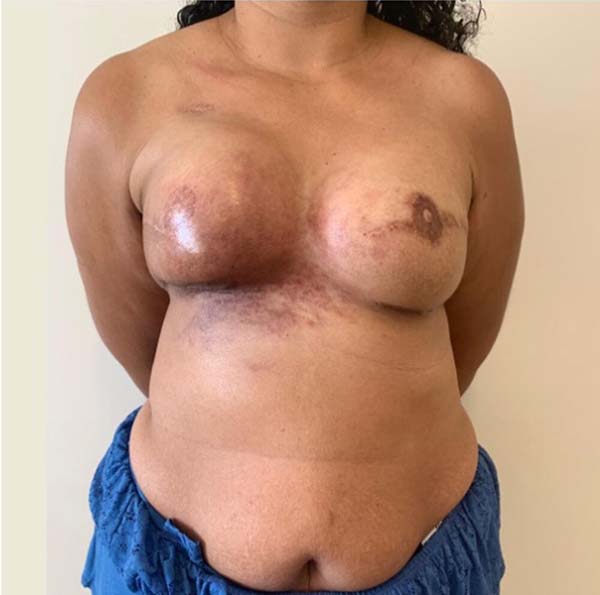
Case 2
A 41-year-old patient with no comorbidities, diagnosed with invasive carcinoma in the left breast, undergoing left mastectomy with sentinel lymph node biopsy (negative) and contralateral prophylactic mastectomy + immediate reconstruction with retromuscular silicone implants with lower pole amputation (Torek) in December 2017, without complications. She underwent adjuvant radiotherapy in the left breast (last session in August 2018), and the second surgical period in June 2019 for left capsulotomy + implant repositioning, fat grafting for symmetrization and correction of scars in axillary extensions (reconstruction of nipple-areola complex programmed in the third period) without complications as shown in Figure 6.
DISCUSSION
As a result of early detection and improvement in treatments such as surgery, hormonal therapies, chemotherapy, and radiotherapy, the mortality rate for breast cancer has been decreasing since the 1950s. Therefore, more patients with breast cancer are surviving and remaining with sequelae that must be treated. In this study, we sought to describe the histological differences of skin, subcutaneous cell tissue, implant capsule and pectoralis major muscle between irradiated and non-irradiated breasts of the same patient. Previous studies have addressed only the skin and subcutaneous region.
The findings of epidermis hyperplasia, flattening of the papillary layer, atrophy of the skin appendages, high density of the skin collagen fibers, and presence of unidirectional collagen fibers had already been reported11,12. Atrophy of skin appendages is of particular clinical importance, as loss of sebaceous tissue and sweat glands lead to dehydrated skin, resulting in the need for long-term skincare using moisturizers. Chronic radiation dermatitis is reported to be associated with fibroblast atypia, which is not seen in other types of fibrosis, such as third-degree burn scars12.13. The present study corroborates these findings. Other findings included the following: reduction of elastic fibers in the deep dermis, vascular congestion in fatty tissue, and hyalinization of vascular walls. Although histological changes in dermatitis due to radiation have already been described12,13, these have been considered to be less important clinically with acceptable side effects. However, this does not apply when planning breast reconstruction with silicone expander/implant.
Archambeau et al., in 199511, found skin changes due to irradiation progressing for up to 10 years. Given this fact, the delay in indicating reconstruction after radiotherapy does not increase safety. This finding could explain the mild findings found in cases 5 and 7, in which the last radiotherapy session was more recent (10 and 8 months, respectively).
One of the main complications of radiotherapy is fibroproliferation of the capsular tissue around the implant with a resulting capsular contracture. This leads to an inadequate expansion with distortion and undesirable aesthetic results, sometimes causing additional surgery. Currently, the pathogenetic mechanism of fibroproliferation and capsular contracture induced by radiation is unknown. The correct anatomical description of the observed changes can help in the development of new studies, unraveling the biochemical mechanisms involved in this pathogenesis.
Understanding the pathogenesis of the fibroproliferative process, which starts with the expansion of the tissue previously subjected to radiotherapy, may probably lead to the discovery of prevention strategies or clinical treatment. For example, COX-2 selective inhibitors are commercially available and were effective in partially decreasing cell proliferation in fibrosis models mediated by increased catenin levels17. There is great potential to explore treatment protocols in an animal model and eventually in clinical trials.
Encapsulation occurs as a result of an inflammatory response to the presence of the foreign body, and fibrosis progresses to nearby tissues. When fibrosis progresses excessively, due to the persistence of the inflammatory response and exposure to external risk factors, contracture occurs around the thickened capsule18.
Therefore, breast reconstruction with implants is performed under the assumption that if radiotherapy is administered, capsular contracture will be recognized as a fundamental limitation, and many studies will be conducted to find solutions to this question.
Kim et al., in 201819, confirmed that the infiltration of myofibroblasts was promoted in irradiated mice, suggesting that this phenomenon acts as a catalyst to accelerate the progression of contracture. We did not find this type of cellular infiltration in any of the samples of irradiated breast.
Some studies reported using coverage with acellular skin matrix and some medications such as montelukast, antileukotrienes, and steroids to reduce the occurrence of capsular contracture around textured implants20,5. Although it is consensus that radiation can induce fibroproliferation in skin and subcutaneous tissues11,13, the relative occurrences of specific molecular mechanisms are still unclear.
We believe that dry skin may be related to atrophy of the skin appendages. The loss of skin elasticity was related to the reduction of elastic fibers in the deep dermis, epidermal hyperplasia, flattening of the papillary layer, high density of skin collagen fibers, and unidirectional alignment of collagen fibers. Hypovascularized fat was related to subcutaneous vascular congestion and hyalinization of vascular wall. The thickened and contracted capsule was related to lower density of elastic fibers, capsular hyalinization, capsular fibrosclerosis, and synovial metaplasia. Hypotrophic and hypovascularized muscle was related to perivascular fibrosis and skeletal muscle sequestration by capsule.
With the data obtained so far, it is not possible to establish a cause and effect relationship, but we will continue to include new patients into the study and try to optimize the quantitative analysis of the information to get to this point. Since each histological evaluation was performed between the breasts of the same patient and not between two different groups, even a small number of patients provided significant results.
CONCLUSION
We found common histological changes in irradiated breasts in most patients. These findings are compatible with the clinical and macroscopic changes observed. This is a descriptive study that presents itself as a pilot for the development of new studies investigating the physiopathological mechanisms related to the described histological changes, thus proposing methods of prophylaxis and treatment for the complications of radiotherapy.
COLLABORATIONS
|
AB |
Analysis and/or data interpretation, Final manuscript approval, Realization of operations and/or trials |
|
RCSD |
Analysis and/or data interpretation, Conception and design study, Data Curation, Writing - Review & Editing |
|
ACC |
Analysis and/or data interpretation |
|
COPC |
Analysis and/or data interpretation |
|
MCC |
Final manuscript approval |
|
JCD |
Final manuscript approval |
REFERENCES
1. Albornoz CR, Bach PB, Mehrara BJ, Disa JJ, Pusic AL, McCarthy CM, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15-23.
2. Polednak AP. Postmastectomy breast reconstruction in Connecticut: trends and predictors. Plast Reconstr Surg. 1999;104(4):669-73.
3. Ji YH, Kim MS, Jung H, Yoo SY, Cho CK. Clinical characteristics of radiation oncology in Korea during past 10 years. J Korean Med Sci. 2009;24:1165-9.
4. Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124(2):395-408.
5. Olbrich KC, Meade R, Bruno W, Heller L, Klitzman B, Levin LS. Halofuginone inhibits collagen deposition in fibrous capsules around implants. Ann Plast Surg. 2005;54(3):293-6;discussion:296.
6. Spear SL, Spittler CJ. Breast reconstruction with implants and expanders. Plast Reconstr Surg. 2001;107:177-87.
7. Iwahira Y, Yamakawa T, Maruyama Y, Saze M. Breast reconstruction using textured, integrated-valve tissue expanders. J Jpn Plast Reconstr Surg. 2004;24:771-8.
8. Malahias M, Jordan DJ, Hughes LC, Hindocha S, Juma A. A literature review and summary of capsular contracture: an ongoing challenge to breast surgeons and their patients. Int J Surg Open. 2016;3:1-7.
9. Ho AL, Bovill ES, Macadam SA, Tyldesley S, Giang J, Lennox PA. Postmastectomy radiation therapy after immediate two-stage tissue expander/implant breast reconstruction: a University of British Columbia perspective. Plast Reconstr Surg. 2014;134(1):1e-10e.
10. Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet. 1983;1(8322):459-62.
11. Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31(5):1171-85.
12. Junkins-Hopkins JM. Disorders associated with physical agents: heat, cold, radiation, and trauma. In: Elder DE, Elenitsas R, Johnson BL, Murphy GE, Xu X, eds. Lever’s histopathology of the skin. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 343-60.
13. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
14. Hoeller U, Borgmann K, Bonacker M, Kuhlmey A, Bajrovic A, Jung H, et al. Individual radiosensitivity measured with lymphocytes maybe used to predict the risk of fibrosis after radiotherapy for breast cancer. Radiother Oncol. 2003;69:137-44.
15. Svensson JP, Stalpers LJ, Lange REE, Franken NA, Haveman J, Klein B, et al. Analysis of gene expression using gene sets discriminates cancer patients with and without late radiation toxicity. PLoS Med. 2006;3(10):e422.
16. Huang, J. et al. Harnessing structural darkness in the visible and infrared wavelengths for a new source of light. NATURE Nanotechnology, p. 60-66, 2016.
17. Spear SL, Onyewu C. Staged breast reconstruction with saline- filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105(3):930-42.
18. Dowden RV. Discussing the advantages of saline and silicone implants in clinical practice. Aesthet Surg J. 2011;31(2):265-6.
19. Kim JB, Jeon HJ, Lee JW, Choi KY, Chung HY, Cho BC, et al. A murine model of radiation-induced capsule-tissue reactions around smooth silicone implants. J Plast Surg Hand Surg. 2018;52(4):217-24.
20. Hester Junior TR, Ghazi BH, Moyer HR, Nahai FR, Wilton M, Stokes L. Use of dermal matrix to prevent capsular contracture in aesthetic breast surgery. Plast Reconstr Surg. 2012;130(5 Suppl 2):126s-36s.
1. Hospital Daher Lago Sul, Brasília, DF, Brazil.
Corresponding author: Ronan Caputi Silva Dias SHTN , Trecho 2, Lote 3, Bloco E, Ap 206, Life Resort, Asa norte, Brasília, DF, Brazil Zip Code: 70800-230. E-mail: ronancaputidias8@gmail.com
Article received: September 8, 2019.
Article accepted: February 22, 2020.
Conflicts of interest: none.











 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter