

Case Reports - Year 2020 - Volume 35 -
Severe complication by irregular use of industrial silicone in a transsexual patient: a case report
Complicação grave do uso irregular de silicone industrial em paciente transexual: relato de caso
ABSTRACT
The use of industrial liquid silicone as a material for aesthetic modification of body contour is a practice that has been carried out clandestine for about 60 years. Currently, most reports come from countries in Asia and South America, and the victims are mainly women and transsexuals. Due to the large number of cases with complications, the use of industrial silicone for aesthetic purposes has never been approved. However, it continues to be applied alone or associated with other products, determining severe local and systemic complications. We report a case of death of a transsexual patient after injecting industrial silicone in the thighs and buttocks.
Keywords: Silicones; Death; Allografts; Necrosis; Transgender people; Body contour.
RESUMO
O uso do silicone líquido industrial como material para modificação estética no contorno corporal é uma prática realizada de forma clandestina há cerca de 60 anos. Atualmente, a maioria dos relatos provém de países da Ásia e América do Sul e as vítimas são principalmente mulheres e transexuais. Devido ao grande número de casos com complicações, o uso do silicone industrial para fins estéticos nunca foi aprovado. Entretanto, continua a ser aplicado isoladamente ou associado a outros produtos, determinando graves complicações locais e sistêmicas. Relata-se um caso de óbito de paciente transexual após injeção de silicone industrial em coxas e glúteos.
Palavras-chave: Silicones; Morte; Aloenxertos; Necrose; Pessoas transgênero; Contorno corporal
INTRODUCTION
The clandestine injection of industrial liquid silicone to modify body contour became popular around 70 years ago when industrial-grade silicone was developed during World War II for military purposes1-3.
Since the publication of Andrews et al., in 19894, showing for the first time the local and systemic complications of liquid silicone in humans, this type of material has had its use contraindicated by the Food and Drug Administration (FDA) and the former Medicines Division (DIMED) in Brazil4,5.
Currently, the majority of victims are women and transsexuals from countries in Asia and South America. Due to the lack of resources for plastic surgery, they end up using unqualified professionals1-3. Despite the prohibitions, the use of industrial silicone for aesthetic purposes continues to be done alone or in association with other products, leading to severe and potentially fatal complications1,6.
OBJECTIVE
To report a case of death after injection of industrial silicone in the buttocks and thighs in a transsexual patient.
CASE REPORT
In this study, we report a healthy transsexual female patient, 24 years old, presenting an injection of 3000ml of industrial liquid silicone in the buttocks and anterolateral thighs. This procedure was performed in a home environment by a non-qualified professional.
After five days, she started showing signs of inflammation and epidermolysis at the infiltration site, being submitted to superficial debridement at a medical service near her residence. Due to a worsening of her general condition, she then sought the emergency room at the Hospital das Clínicas of the University of São Paulo.
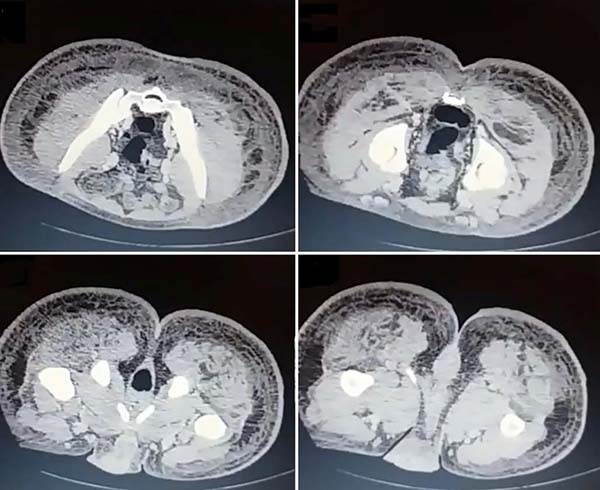
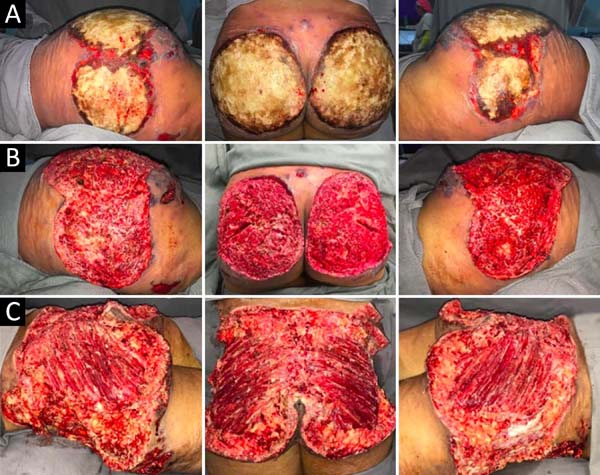
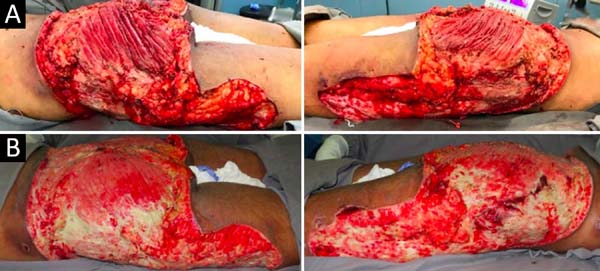
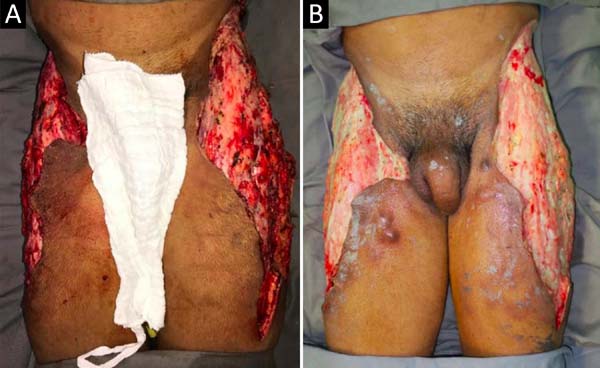
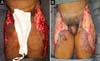
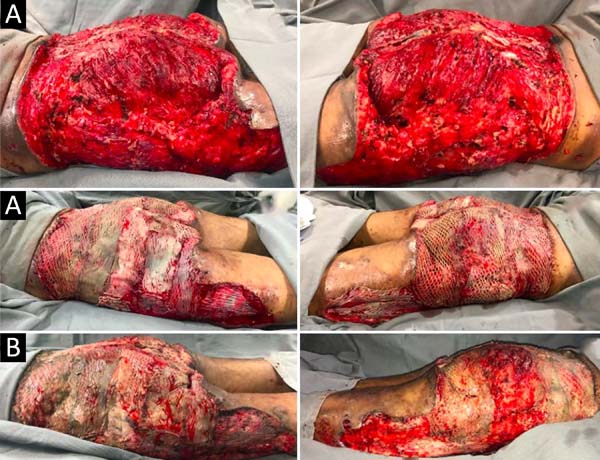
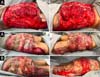
Upon admission, she already had extensive necrosis in the glutes and lateral region of the hip associated with signs of septic shock, requiring orotracheal intubation and the use of vasoactive drugs. Imaging exams showed diffuse densification with liquid laminae pervaded, more pronounced in the lumbar, sacral, buttocks, and thigh roots (Figure 1). The patient underwent six sequential surgical procedures for extensive debridement of devitalized tissues, with identification of purulent collections and viscous substance, compatible with silicone (Figures 2, 3, and 4). Initially, negative pressure therapy was used. After the second debridement, it was replaced by a simple dressing with 1% silver sulfadiazine and cerium nitrate. Cultures guided antibiotic therapy.
With a condition of acute renal failure attributed to sepsis and the use of nephrotoxic drugs, the patient remained in the ICU.
On the thirty-second day of hospitalization, because of an apparent local and systemic control of the infection, partial allogeneic skin grafting was performed, in mesh (3: 1), on the raw areas to reduce the degree of spoliation (Figure 5).
However, five days after the grafting, the patient presented a new clinical worsening with hemodynamic instability, evolving to death. The necropsy report defined the cause of death as a septic shock with pulmonary and skin focus.
DISCUSSION
Polydimethylsiloxane (silicone) is a compound formed by the conjugation of silicon with oxygen and methane. In its manufacture, it is inherently contaminated with impurities, heavy metals, and volatile polymers. Besides, when it hardens, it ends up releasing acetic acid, which may be responsible for the initial tissue damage after the injection. This combination of factors contributes to the severe complications frequently observed6.
In addition to its isolated use, silicone is also intentionally associated with other agents to increase inflammation and fibroplasia at injection sites, preventing its migration by gravitational action. Sakurai’s formula is a well-known example of its association with olive oil. Other sclerosing agents used are croton oil, snake venom, and peanut oil7.
Winer et al. created the term siliconoma., in 19642, to describe the foreign body reaction similar to those already described after the injection of oil and paraffin. These substances promote an equivalent type of anatomopathological tissue reaction, called sclerosing lipogranulomatosis1,5,8,9.
In an attempt to eliminate, through the phagocytic activity of tissue macrophages and circulating blood cells, the silicone can be transported by the lymphatic route to organs at a distance, leading to embolism. Besides, its intravascular injection can also result in immediate embolism4,10,11.
Due to the illegal nature of the practice, there are few reports of acute reactions in this context. These patients are reluctant to seek medical attention, except in life-threatening circumstances. The most severe systemic manifestations include pulmonary, neurological, cardiac, hepatic, gastrointestinal involvement and sepsis12.
From a local point of view, complications range from skin color and consistency changes to an intense inflammatory process with nodules, ulceration, necrosis, abscesses, and fistulas. Scarring retractions and deformities are also observed. The latency period for these sequelae appearance is variable, reaching up to 30 years. Therefore, identifying and punishing those responsible is often difficult5,10.
According to the literature, the complete elimination of silicone deposits is not feasible, since liquid silicone diffuses through deep tissues, forming islands of fibrosis among healthy tissues. Thus, its eradication would culminate in very extensive resections leading to even more severe sequelae3,5,9.
The debridement of devitalized tissues and early irrigation can minimize the damage caused by the initial silicone hardening reaction and dilute contaminants. In addition to surgical intervention, the use of antimicrobial dressings, intravenous antibiotics, and systemic steroids is also recommended5,9.
Allogeneic skin grafting, as a biological dressing, is an option until the wound bed is appropriately prepared to receive autografts or other definitive coverage. Local or regional flaps should be used to rebuild areas with exposure to deep structures.
Despite reports of adjuvant therapies such as hyperbaric oxygen, intralesional corticosteroids, and topical immunomodulators, there are not yet enough studies validating their effectiveness. Liposuction does not seem to be effective in removing tissues impregnated with fibrous oil. The intense local fibrosis alone makes aspiration with cannulas difficult and increases the risk of injury to adjacent structures3,5.
The National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária, Anvisa) prohibits the use of industrial-grade liquid silicone in cosmetic procedures, and its application is considered a crime against public health provided for in the Penal Code. For aesthetic purposes, polydimethylsiloxane (silicone) is the raw material for many prostheses and implants and must be handled by qualified people and in a hospital environment13.
The exclusive use of the medical product containing silicone oil authorized by Anvisa is for the treatment of diseases of the retina to promote intraocular tamponade9,14. Therefore, its use is restricted to the doctor specialized in ophthalmology and is prohibited for facial fillings or body contour treatment15.
CONCLUSION
The injection of industrial liquid silicone for aesthetic purposes to alter body contour is strongly contraindicated and is considered a crime against public health provided for in the Penal Code. Its misuse produces serious complications, challenging to treat and potentially fatal, as described in this case report.
REFERENCES
1. Behar TA, Anderson EE, Barwick WJ, Mohler JL. Sclerosing lipogranulomatosis: a case report of scrotal injection of automobile transmission fluid and literature review of subcutaneous injection of oils. Plast Reconstr Surg. 1993 Fev;91(2):352-61.
2. Winer LH, Sternberg TH, Lehman R, Ashley FL. Tissue reactions to injected silicone liquids. A report of three cases. Arch Dermatol. 1964 Dez;90:588-93.
3. Hage JJ, Kanhai RC, Oen AL, van Diest PJ, Karim RB. The devastating outcome of massive subcutaneous injection of highly viscous fluids in male-to-female transsexuals. Plast Reconstr Surg. 2001 Mar;107(3):734-41.
4. Andrews JM, Haddad CM, Ramos RR, Martins DMFS, Ferreira LM. Morbidade e mortalidade após injeção de silicone líquido em seres humanos. A Folha Médica. 1989 Ago;99(2).
5. Freitas RJ, Cammarosano MA, Rossi RHP, Bozola AR. Injeção ilícita de silicone líquido: revisão de literatura a propósito de dois casos de necrose de mamas. Rev Bras Cir Plást. 2008;23(1):53-7.
6. Chasan PE. The history of injectable silicone fluids for soft-tissue augmentation. Plast Reconstr Surg. 2007;120(7):2034-40;discussion:2041-3.
7. Balkin SW. DPM injectable silicone and the foot: a 41-year clinical and histologic history. Dermatol Surg. 2005;31:1557.
8. Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006 Set;118(3 Suppl):77S-84S.
9. Rohrich RJ, Potter JK. Liquid injectable silicone: is there a role as a cosmetic soft-tissue filler?. Plast Reconstr Surg. 2004 Abr;113(4):1239-41.
10. Gemperli R, Alonso N, Lodovici O, Pigossi N. Estudo clínico das reações sistêmicas e locais ao uso indevido do silicone líquido e/ou óleo mineral. Rev Hosp Fac Med S Paulo. 1984;39(4):158-62.
11. Schmid A, Tzur A, Leshko L, Krieger BP. Silicone embolism syndrome: a case report, review of the literature, and comparison with fat embolism syndrome. Chest. 2005 Jun;127(6):2276-81.
12. Bartsich S, Wu JK. Silicone embolism syndrome: a sequela of clandestine liquid silicone injections. A case report and review of the literature. J Plast Reconstr Aesthet Surg. 2010;63(1):e1-3.
13. Agência Nacional de Vigilância Sanitária (ANVISA). Portal ANVISA [Internet]. Brasília (DF): ANVISA; 2020; [acesso em 2020 Mar 10]. Disponível em: http://portal.anvisa.gov.br/
14. Siqueira RC, Gil ADC, Jorge R. Cirurgia de descolamento de retina com injeção de óleo de silicone no sistema de vitrectomia transconjuntival sem sutura de 23-gauge. Arq Bras Oftalmol. 2007;70(6):905-9.
15. Agência Nacional de Vigilância Sanitária (ANVISA). Consultas [Internet]. Brasília (DF): ANVISA; 2020; [acesso em 2020 Fev 10]. Disponível em: https://consultas.anvisa.gov.br/#/saude/253510028760211/
1. Hospital das Clínicas, Faculty of Medicine, University of São Paulo, São Paulo,
SP, Brazil.
Institution: Hospital das Clínicas, Faculty of Medicine, University of São Paulo, São Paulo, SP, Brazil.
MM Analysis and/or data interpretation, Conceptualization, Data Curation, Final manuscript approval, Methodology, Visualization, Writing - Original Draft Preparation
GGRM Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Formal Analysis, Methodology, Project Administration, Writing - Original Draft Preparation, Writing - Review & Editing
EfBB Conception and design study, Data Curation, Final manuscript approval, Formal Analysis, Methodology, Writing - Original Draft Preparation
DAM Analysis and/or data interpretation, Conception and design study, Final manuscript approval, Formal Analysis, Methodology, Supervision, Validation, Writing - Original Draft Preparation
AAMJ Final manuscript approval, Supervision, Validation, Writing - Review & Editing
RG Analysis and/or data interpretation, Supervision, Writing - Review & Editing
Corresponding author: Gustavo Gomes Ribeiro Monteiro, Rua Enéas de Carvalho Aguiar 255, Ribeirão Preto, SP, Brazil. Zip Code: 14020-130. E-mail: monteiroggr@hotmail.com
Article received: April 14, 2019.
Article accepted: June 12, 2020.
Conflicts of interest: none.




 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter