

Original Article - Year 2020 - Volume 35 -
Cultural adaptation of the questionnaire on skin rejuvenation outcome evaluation (Skin Rejuvenation Outcome Evaluation - SROE)
Adaptação cultural do questionário de avaliação de resultados em rejuvenescimento de pele (Skin Rejuvenation Outcome Evaluation - SROE)
ABSTRACT
Introduction: The use of quality of life (QOL) questionnaires
has been shown to be very useful in the sense of giving
greater objectivity to the treatment outcomes evaluation.
The internationalization of these instruments, in turn,
allows for inter-population comparison but requires a
specific methodology in order not to cause distortions due
to flaws in translation or cultural differences. The SROE
questionnaire (Skin Rejuvenation Outcome Evaluation), in
English, is a simple application tool, with objective questions
with a good application for this purpose. The questionnaire
has already been tested for its reliability, validity, and
responsiveness.
Methods: Translation and cultural adaptation
to the Portuguese language was carried out, according to
the methodology proposed by Beaton et al. (2000), in which
there are five stages: stage 1 - translation using two native
Portuguese-speaking translators; stage 2 - making a synthesis
version; stage 3 - reverse translation by two native Englishspeaking
translators; stage 4 - review by an evaluation
committee; and, stage 5 - application to a population of
20 people.
Results: The questionnaire was successfully
translated and adapted, without problems of understanding
for the final population.
Conclusion: Considering the applied
methodology, we conclude that the SROE questionnaire's
translation is suitable for use in Brazilian Portuguese.
Keywords: Rejuvenation; Surveys and questionnaires; Skin; Translational medical research; Outcome assessment (health care).
RESUMO
Introdução: O emprego de questionários de qualidade de vida (QV) tem se mostrado muito útil no sentido de dar maior objetividade à avaliação de resultados de tratamentos. A internacionalização desses instrumentos, por sua vez, permite a comparação interpopulacional, mas requer uma metodologia específica, a fim de não causar distorções devido a falhas na tradução ou a diferenças culturais. O questionário SROE (Skin Rejuvenation Outcome Evaluation), de língua inglesa, é uma ferramenta de simples aplicação, com perguntas objetivas com boa aplicação para esse fim. O questionário já foi testado em relação à sua confiabilidade, validade e capacidade de resposta.
Métodos: Realizada tradução e adaptação cultural para a língua portuguesa, segundo a metodologia proposta por Beaton et al. (2000), na qual existem 5 estágios: estágio 1 - tradução por meio de dois tradutores nativos de língua portuguesa; estágio 2 - confecção de versão de síntese; estágio 3 - tradução reversa por dois tradutores nativos de língua inglesa; estágio 4 - revisão por um comitê avaliador; e, estágio 5 - aplicação a uma população de 20 pessoas.
Resultados: O questionário foi traduzido e adaptado com sucesso, sem problemas de compreensão para a população final.
Conclusão: Considerando a metodologia aplicada, concluímos que a tradução do questionário SROE é adequada para utilização em língua portuguesa do Brasil.
Palavras-chave: Rejuvenescimento; Inquéritos e questionários; Pele; Pesquisa médica translacional; Avaliação de processos e resultados (cuidados de saúde)
INTRODUCTION
The search for greater objectivity in evaluating treatment outcomes is necessary to improve evidence levels, especially in plastic surgery, whose major purpose is a subjective parameter, to enhance life quality (QOL)1.
In the literature, it is clear that cosmetic procedures’ objective measurement mechanisms are still in their infancy. Anyway, they point to the trend of using patient reported outcome measures (PROM or PRO) through questionnaires, according to Morley 20122. Fortunately, there are already significant advances in this area, with the publication of several articles proposing questionnaire models4.
The Breast-Q, Face-Q, and Satisfaction with Facial Appearance Scale and Skindex tools, for example, have already undergone a rigorous validation process, are entirely in line with the acceptance requirements of the US Food and Drug Administration (FDA) and stand out, together with Skindex, in relation to the other PROMs, according to Morley 20122. Kosowski et al., in 20094, found 442 articles evaluating outcomes in aesthetic, surgical or non-surgical procedures. Among these, 47 were specific to facial appearance, but only 9 met the study’s inclusion and exclusion criteria. None of them met all the guidelines. All tools proved to be limited, either due to their development, validation, or content. In that same study, Skin Rejuvenation Outcome Evaluation (SROE) and two other articles were specific for skin rejuvenation4.
The SROE (Figure 1) was described by Alsarraf, in 20005, together with other questionnaires in English specific to blepharoplasty (Blepharoplasty Outcome Evaluation - BOE), rhinoplasty (Rhinoplasty Outcome Evaluation - ROE), and rhytidoplasty (Facelift Outcome Evaluation - FOE).
Such questionnaires address the physical, mental and social aspects, necessary for a fair assessment. The SROE questionnaire was tested concerning its validity, reliability, and responsiveness, being presented as a reliable quantitative tool for measuring outcomes3,6,7.
The internationalization of these questionnaires, in turn, allows the comparison of treatment outcomes between different populations. However, some care must be taken so that there are no distortions due to flaws in the translation or cultural differences that alter the questions’ result. This would decrease the inter-population comparative value8,9. Of the four questionnaires by Alsarraf (2000)5, only the ROE (Rhinoplasty Outcome Evaluation) and FOE (Facelift Outcome Evaluation) have been translated into Portuguese so far. The others, Blepharoplasty Outcome Evaluation (BOE) and Skin Rejuvenation Outcome Evaluation (SROE), do not have Portuguese versions yet10,11.
The SROE consists of six questions, as shown in Figure 1. Each answer can be rated from 0 (least satisfied as possible) to 4 (most satisfied as possible). The marked values must be added, divided by 24, and multiplied by 100, to obtain a score from 0 to 100, with 0 being the least satisfied possible and 100 the most satisfied5.
Such an instrument can be of great use for developing scientific studies and monitoring outcomes by surgeons and dermatologists. Thus, the present study’s objective is to translate and culturally adapt the SROE questionnaire to Brazilian Portuguese.
OBJECTIVE
Translate and culturally adapt the Skin Rejuvenation Outcome Evaluation (SROE) questionnaire to Brazilian Portuguese.
METHODS
The study was authorized by the Research Ethics Committee of the Universidade Federal do Ceará, under protocol number 33290513.8.0000.5589 and performed at the clinic Eduardo Furlani Cirurgia Plástica in 2019.
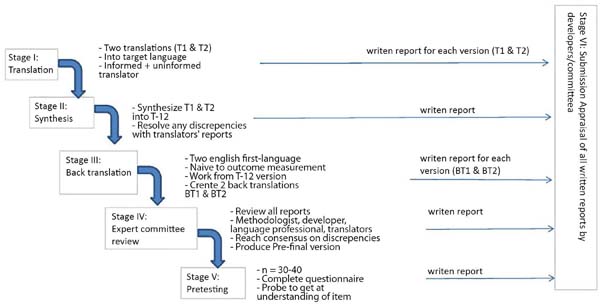
The SROE questionnaire was translated and culturally adapted to the Portuguese language of Brazil, according to the methodology proposed by Beaton et al. (2000)8, according to the flowchart in Figure 2. This methodology consists of five stages and is accepted in the literature for translation of several other instruments.
Translation
Stage 1: the questionnaire was submitted to two translations (T1 and T2) from English to Portuguese. One was performed by a lay translator and the other by a plastic surgeon translator, experienced in the procedure, as recommended by the literature.
Stage 2 (synthesis): the Portuguese versions T1 and T2 of the questionnaires were evaluated by both stage 1 translators. The translators discussed the differences between their versions and developed a consensual version, called T-12.
Stage 3 (reverse translation): the T-12 questionnaire was submitted to two lay translators (B1 and B2), unaware of each other or of the current study, whose native language was English.
Stage 4 (submission to a specialist committee): a medical board with knowledge in the area was requested to follow the process, evaluating the versions, pointing out inconsistencies and deviations. This board was composed of a dermatologist, a general surgeon, and an orthopedist.
There was pacification, through discussions, on four points:
Semantic equivalence: the translations were evaluated to preserve their meaning, for the possibility of multiple meanings, and the existence of grammatical difficulties.
Idiomatic equivalence: expressions or colloquialisms are difficult to translate. The committee sought the presence of these expressions and equivalents in the Portuguese language.
Experimental equivalence: the questioned experiences were evaluated for their existence in the Portuguese language.
Conceptual equivalence: expressions must contain the same concept. For example, when it comes to the family, in some cultures, it has the meaning of the nearest small family unit, and others include all relatives.
Cultural adaptation
Stage 5 (or pre-final version test): a pre-test with the final version T-12 was carried out with a sample of 20 people. This group was composed of graduate students of dermatofunctional physiotherapy. Each participant completed the questionnaire and was interviewed by the researcher to point out possible inconsistencies and difficulties in understanding.
Stage 6: submission of documentation to the expert committee to verify the translation process.
RESULTS
The SROE questionnaire was translated into versions T1 and T2; there were some divergence points between the two versions, but a consensus was reached on the T-12 version. This version was subjected to two reverse translations B1 and B2, which presented some divergences but without changing the original meaning.
Versions B1 and B2 were analyzed by the author of the original questionnaires, contacted by e-mail, who did not identify any change in meaning or inconsistency between the questionnaire translated from Portuguese into English and its original version.
There were no difficulties in completing and understanding the questionnaires.
The committee evaluated all steps of the translation process and contributed suggestions for changes, which were accepted. Consequently, the final result of the translation was as follows (Figure 3).
DISCUSSION
There were no significant difficulties in translating the questionnaires due to the small number of expressions with no known translation into Portuguese.
We believe that quality of life questionnaires are essential to make some subjective parameters more objective and comparable. This allows outcomes to be compared, providing better levels of evidence in an area of knowledge that lacks it. However, there is still no perfect questionnaire.
The comparison of tools is outside the scope of this study. However, although the SROE questionnaire has shown its validity, reliability, and responsiveness statistically, its design does not seem to be as well-founded as that of Face-Q, which provides a significant interval level. This allows the construction of defined units with a uniform distance between them. For example, this means that if a person evolved from a score of 100 to 120, there was an increase similar to that of another who grew from 120 to 140, which is not true for most other instruments12.
Unlike Face-Q, the SROE is a specific questionnaire for non-surgical, public, and freely available skin rejuvenation, facilitating its translation. Its application takes less than 1 minute. Other shortcomings of SROE are that there are still no cut-off points and normality levels, even in their original language, which we suggest for future studies.
CONCLUSION
Considering the applied methodology, we conclude that the SROE questionnaire’s translation is suitable for use in Brazilian Portuguese.
REFERENCES
1. Ferreira MC. Cirurgia plástica estética: avaliação dos resultados. Rev Soc Bras Cir Plást. 2000 Jan/Abr;15(1):55-61.
2. Morley D, Jenkinson C, Fitzpatrick R. A structured review of patient reported outcome measures used for cosmetic surgical procedures. Report to Department of Health [acesso 02 Fev 2015]. Disponível em: http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Elective%20Cardiac_012011.pdf
3. Davies N, Fitzpatrick R, Gibbons E, Mackintosh A. A structured review of patient-reported outcome measures used in elective procedures for coronary revascularisat ion. Patient-Reported Outcome Measurement Group. Oxford: University of Oxford; 2009.
4. Kosowski TR, McCarthy C, Reavey PL, Scott AM, Wilkins EG, Cano SJ, et al. A systematic review of patient-reported outcome measures after facial cosmetic surgery and/or nonsurgical facial rejuvenation. Plast Reconstr Surg. 2009 Jun;123(6):1819-27.
5. Alsarraf R. Outcomes research in facial plastic surgery: a review and new directions. Aesthetic Plast Surg. 2000 Mai/Jun;24(3):192-7.
6. Alsarraf R, Larrabee WF, Anderson S, Murakami CS, Johnson Junior CM. Measuring cosmetic facial plastic surgery outcomes: a pilot study. Arch Facial Plast Surg. 2001 Jul/Set;3(3):198-201.
7. Alsarraf R. Outcomes instruments in facial plastic surgery. Facial Plast Surg. 2002;18(2):77-86.
8. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross- cultural adaptation of self-report measures. Spine. 2000 Dez;25(24):3186-91.
9. Ferreira LF. Tradução para a língua portuguesa, adaptação cultural e validação do Breast Evaluation Questionnaire [dissertação]. São Paulo (SP): Universidade Federal de São Paulo (UNIFESP); 2009.
10. Izu SC, Kosugi EM, Brandão KV, Lopes AS, Garcia LBS, Suguri VM, et al. Normal values for the Rhinoplasty Outcome Evaluation (ROE) questionnaire. Braz J Otorhinolaryngol. 2012 Jul/Ago;78(4):76-9.
11. Furlani EAT. Cultural adaptation of rhytidectomy outcome evaluation questionnaire: facial outcome evaluation. Rev Bras Cir Plást. 2015;30(3):501-5.
12. Pusic AL, Klassen AF, Scott AM, Cano SJ. Development and psychometric evaluation of the FACE-Q satisfaction with appearance scale: a new patient-reported outcome instrument for facial aesthetics patients. Clin Plast Surg. 2013 Abr;40(2):249-60.
1. Clínica Eduardo Furlani, Fortaleza, CE, Brazil.
EATF Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Formal Analysis, Funding Acquisition, Methodology, Project Administration, Realization of operations and/or trials, Resources, Supervision, Visualization, Writing - Original Draft Preparation, Writing - Review & Editing
DBS Formal Analysis, Visualization, Writing - Review & Editing
Corresponding author: Eduardo Antonio Torres Furlani, Rua Barbosa de Freitas, 1990, Aldeota, Fortaleza, CE, Brazil. Zip Code: 60170-021. E-mail: eduardo@eduardofurlani.com.br
Article received: April 20, 2020.
Article accepted: July 19, 2020.
Conflicts of interest: none








 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter