

Original Article - Year 2021 - Volume 36 -
Thermoguided technique of lipolysis and skin retraction with 980nm diode laser
Técnica termoguiada de lipólise e retração da pele com laser diodo 980nm
ABSTRACT
Introduction: Liposuction is a consecrated procedure to improve body contouring, and various types of laser can be applied to cause lipolysis and skin retraction. The current parameters (power and energy) in the devices allow only an indirect inference of skin temperature change.
Methods: Eighty-three patients, between 17 and 75 years old, were submitted to laser lipolysis with 980nm diode, using a Flir® T540 thermocamera for temperature monitoring. Lipolysis was obtained by applying the laser to the deep subcutaneous layer and skin retraction in the deep dermis, with a velocity of 5 centimeters per second. The laser was used in the deep dermis of the abdominal flaps of patients undergoing dermolipectomy to determine the temperature that causes skin burns, totaling 27 cases.
Results: A power of 15W was used on the medial side of the arm and thigh, and 4000J average energy was applied per region. In the dorsal area and abdomen, 20W was adopted, and 6000J were applied in each quadrant. On average, the studied tissue's initial temperature was 31°C, and at the end of the laser application was 37°C. The temperature that caused the burn was 45°C.
Conclusion: The use of the thermocamera allowed a more homogeneous distribution of the laser and provided the surgeon with effective monitoring so that an adequate dose of energy is applied to the subcutaneous and deep dermis, obtaining the desired effect.
Keywords: Lipectomy; Laser; Thermography; Subcutaneous fat; Skin.
RESUMO
Introdução: A lipoaspiração é um procedimento consagrado para melhora do contorno corporal, sendo que existem diferentes tipos de laser que podem ser aplicados com o objetivo de provocar lipólise e retração da pele. Os parâmetros existentes atualmente (potência e energia) nos aparelhos permitem apenas uma inferência indireta da mudança de temperatura da pele.
Métodos: Oitenta e três pacientes, entre 17 e 75 anos, foram submetidos à laserlipólise com diodo 980nm, utilizando uma termocâmera Flir® T540 para monitorização da temperatura. A lipólise foi obtida pela aplicação do laser na camada subcutânea profunda e a retração da pele na derme profunda, com velocidade de 5 centímetros por segundo. Para determinar a temperatura que provoca queimadura na pele, o laser foi aplicado na derme profunda dos retalhos abdominais das pacientes submetidas à dermolipectomia, totalizando 27 casos.
Resultados: Na face medial do braço e da coxa foi utilizada uma potência de 15W e foram aplicados em média 4000J de energia por região. Na região dorsal e abdome foi adotada potência de 20W e foram aplicados 6000J em cada quadrante. No submento foram utilizados 10W e aplicados 1500J. Em média, a temperatura inicial do tecido estudado foi de 31°C, e ao final da aplicação do laser foi de 37°C. A temperatura que provocou queimadura foi 45°C.
Conclusão: A utilização da termocâmera permitiu uma distribuição mais homogênea do laser e propiciou ao cirurgião uma monitorização eficaz, para que seja aplicada no subcutâneo e na derme profunda uma dose adequada de energia, obtendo-se o efeito desejado.
Palavras-chave: Lipectomia; Laser; Termografia; Gordura subcutânea; Pele
INTRODUCTION
Liposuction is a procedure consecrated in plastic surgery for the improvement of body contouring. It is the second most performed cosmetic plastic surgery in the country1. It was first described for the treatment of lipodystrophy by French surgeon Illouz in 19832 and has since undergone several technical developments.
The treatment principle is based on removing subcutaneous tissue (content) from the regions affected with the preservation of the skin (continent). After vacuum removal of excess fat, it is assumed that the skin, from its retraction capacity (elasticity), will adapt to this volume reduction of the subcutaneous tissue. However, this skin adaptation may not occur, especially in areas with smaller dermis thickness, such as the thighs and arms’ medial face. This can imply an unwanted side effect, sagging skin, and removal of excess skin with the creation of a scar.
In an attempt to solve this issue, different technologies have been developed to stimulate collagen production and provide more significant skin retraction, such as local injections of poly-L-lactic acid or calcium hydroxyapatite, micro-focused ultrasound applications, radiofrequency electrical current applications, and laser use.
Different laser types can be applied to the subcutaneous tissue and deep dermis to cause lipolysis and skin retraction3. The laser with a wavelength of 980nm has an excellent affinity for the water contained in adipocytes and skin. Therefore, it is effective for lipolysis4 and as a skin retraction stimulator. In Brazil, this technology has been used for some years. In 2013, Dornelles et al. 5 published a series of 400 patients submitted to laser lipolysis utilizing this technology and obtaining good results.
When applied in the subcutaneous and deep dermis region, the laser has a photochemical (alteration of the adipocyte membrane’s permeability) and photothermal through heat production, which generates cellular damage to adipocytes and leads to remodeling of skin collagen 5.
The surgeon who uses this technology is concerned that the production of heat at the site causes a burn of the tissues. The current parameters (power and energy) in the devices allow only an indirect inference in skin temperature change. Therefore, the surgeon needs to choose between applying a laser underdose to the tissue without obtaining the expected effect or causing an unwanted burn on site, without having the precise instruments for this decision making.
Clinical thermography is used in several areas of knowledge currently with applications in the war industry, construction, and safety. In medicine, the first article was published in 19576 and today has several applications such as screening of passengers with infectious diseases at airports7, quantitative and qualitative evaluation in the treatment of pain 8, the quantification of brown fat9, which can be used to monitor inflammatory reactions in orthopedics and rheumatology10, prevention of injuries in sports medicine11 and for the detection of tumors such as melanomas and their metastasis12. In plastic surgery, it has already been used in the preoperative planning of perforating skin flaps, postoperative follow-up of flaps as a complement in the analysis of the depth of burns, in evaluating the hemangioma treatment response, and as a diagnostic test of carpal tunnel syndrome13. However, to date, it has not been described to guide laser liposuction procedures.
There are two types of thermographic evaluation: passive and active - also known as dynamics. In the passive are captured radiometric images with static measurements of the surface in real-time and at a specific instant. In dynamics, thermography assesses the temperature of a surface subject to temperature changes. It measures dynamic physiological responses to stimuli, such as cold and heat, documented over a time interval. Dynamic evaluation is used in provocative tests such as brachial occlusion to evaluate the endothelium14, or the so-called cold stress test or cold challenge test, which activates the autonomic nervous system through exposure to environmental cold or specific limbs, applied in cases of chronic pain and neurological evaluation15,16.
OBJECTIVES
This work aims to describe a new standardized dynamic thermography technique to determine the epidermis temperature in real-time during laser application and, consequently, guide the surgeon, allowing a thermo-guided procedure, increasing efficiency, and reducing the risk of complications.
A second objective is to use this standardization to determine the upper-temperature limit with the method, through the temperature that, on average, burns on the skin during laser application.
METHODS
Eighty-three patients were submitted to laserlipolysis with the Orlight® Duo 980nm diode laser (Figure 1), between July 2017 and June 2019, at the Hospital da Plástica, São Paulo/SP. All patients in the study signed the consent form, and the study received approval from the ethics committee under number 0126/2019. The age range of the patients ranged from 17 to 75 years. All patients were female.
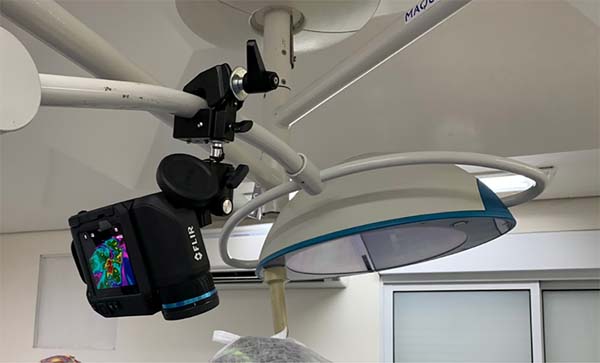
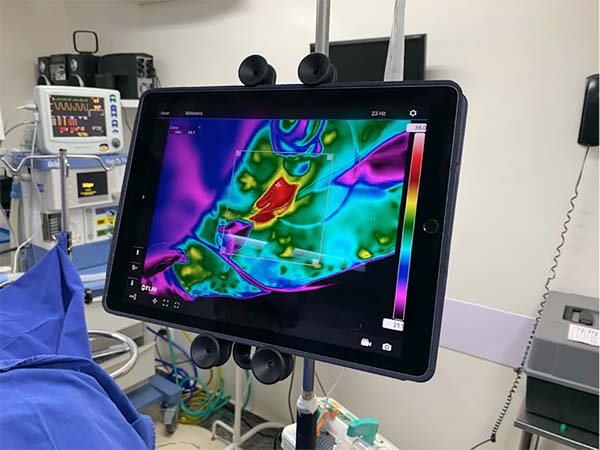
For temperature monitoring, a Flirthermocamera was used ® model T540sc (Flir Systems Inc, Wilsonville, OR) with IR resolution of 464x248 pixels and thermal sensitivity of 30mk and a lens of 42º (Figure 2) fixed to the surgical focus to allow its manipulation by the surgeon himself in a sterile manner (Figure 3). Bluetooth transmitted the images of the thermocamera to an Ipad Pro 12.9’’ tablet fixed in front position to the surgeon (Figure 4).
The temperature and humidity environment (always less than 60%) was controlled with an HTC-1 thermohygrometer ensuring the patient’s thermal comfort who had exposed only the body area to be operated. The patients were not exposed to air currents, and the temperature was maintained at 22ºC.
An alarm was set in the thermocamera when the skin temperature reached 38ºC. Laser application was used to cause skin retraction was made with the tip directed against the deep dermis at the linear 5 centimeters speed every 1 second.
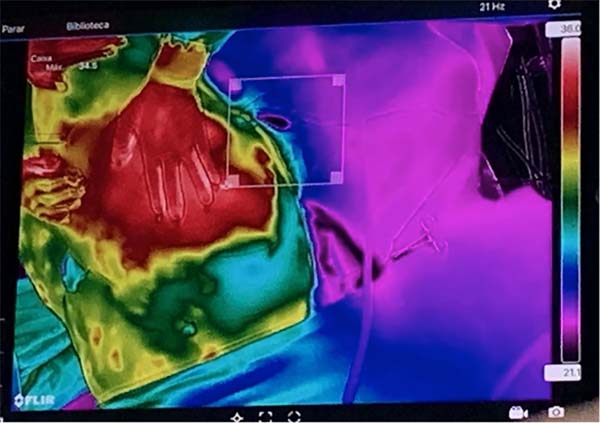
The laser was applied to the tissue until a homogeneous temperature surface was obtained, observed in the thermocamera as a red color throughout the demarcated area (Figure 5).
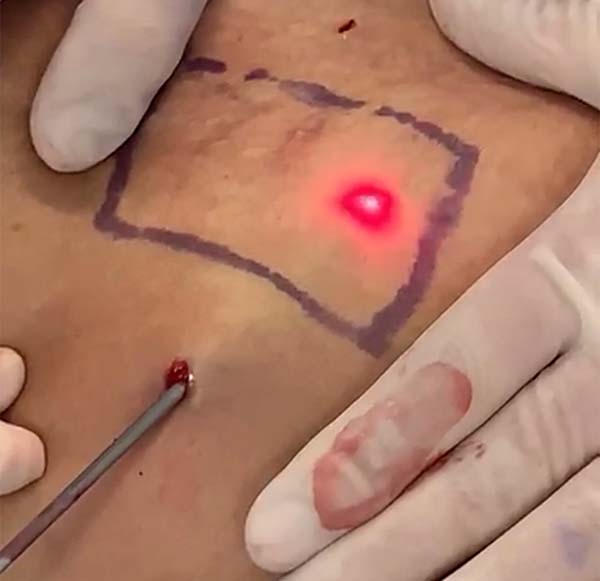
The laser was applied to the deep dermis of abdominal flaps of patients undergoing dermolipectomy to determine the temperature that causes skin burns, totaling 27 cases. According to a previously standardized back table technique, the temperature was measured immediately after resectioning the abdominal flaps, with marked areas of 6x6cm bilaterally (Figure 6).
The skin’s burn temperature was defined as the exact moment of the appearance of an opaque plaque on the surface of the skin, followed by a defect of a total thickness (Figure 7).
RESULTS
It was applied in the arm and thigh’s medial face, 15W, and an average of 4000J of energy per region. In the dorsal and abdomen area, 20W was adopted, and 6000J of energy was used in each quadrant.
In the subment region, 10W was adopted, and an average of 1500J of energy was applied (Table 1).
| Region | Power (w) | Energy |
|---|---|---|
| Medial arm face | 15 | 4000 |
| Medial thigh face | 15 | 4000 |
| Back | 20 | 6000 |
| Abdomen | 20 | 6000 |
| Subment | 10 | 1500 |
The initial temperature of the studied tissue was, on average, 31ºC, with the temperature of the room standardized at 22ºC. The average temperature at the end of laser application was 37ºC (Table 2).
The average temperature that caused the burn was 45ºC, and the safety interval in the thermoguided technique was fixed between 36 and 40º C, adopted as the goal of laser application.
Nine cases with seroma and the need for punctures in the office were verified, and two patients presented minor burns in the subment region, treated conservatively.
DISCUSSION
The generation of heat in the deep dermis region promotes collagen production, activation of the healing process, contraction of collagen, and thickening its fibers17. For this effect to occur correctly, heat must cause intra- and intermolecular rupture of collagen fibrils, which only occurs within a certain temperature range18. Previous studies have shown that this temperature ranges from 60 to 65c19.
Various technologies use the principle of temperature increase to induce this biological effect, such as radiofrequency, ultrasound, and laser20. However, excessive heat can cause burns. According to Lawrence and Bull, in 197621, an object in contact with the skin for any time duration should not exceed 42°C temperature because, at temperatures above 43.5°C, there is tissue damage. Therefore, it is essential to monitor the temperature in the epidermis so that it remains within a safe range, and at the same time, heat induces remodeling of the dermis. Among the different methods used for this purpose, infrared thermography stands out.
Infrared medical thermography is a non-invasive and non-radioactive method of analysis, capable of monitoring the epidermis’ temperature without contact. In 1964, the first record of contactless thermography in evaluating skin burns with gradients up to 0.1ºC was published, allowing the estimating of relative perfusion22. In theory, infrared radiation emitted from a burn wound should decrease according to increased burn depth due to the higher degree of microvascular coagulation, and, consequently, thermography was also applied to calculate the depth of the lesion23,24.
However, static or passive thermography is subject to several factors that can negatively impact the intratemporal result, such as evaporative heat or heating and cooling effects, also wound granulation that modifies the level of perfusion or even emissivity, as well as variations in the depth of vascularization in the cutaneous tissue in different places of the body25.
New dynamic thermography techniques were proposed, where thermal energy was introduced into the skin, raising its physiological temperature. Following the skin temperature in the lesion in time, regional variations in heat energy transfer can be detected on the wound surface, allowing the identification of the burn depth26,27.
In dynamic thermography for the evaluation of deep burns, the temperature response is evaluated after a thermal pulse excitation: initially, the fixed temperature distribution on the surface of interest is measured with an infrared camera; in the sequence is applied the external thermal excitation, following by various temperature measurements on the tested surface for a specific time; finally, the depth of the skin burn can be evaluated quantitatively by calculating the constant T of the thermal time26.
Thermal imaging shows surface skin burns an increase in temperature compared to the nearby healthy area, while deep burns have lower temperatures than nearby healthy regions. This temperature difference is statistically significant and provides a way to distinguish superficial skin burns from deep burns28.
In this research, we used a thermography camera, which transmits the infrared image in real-time to Bluetooth’s monitor. Allows thermal monitoring of the entire treated region, unlike a thermometer that only one specific point at a time. To have a comparison parameter, when using an infrared camera with a resolution of 464×348 pixels, this is equivalent to 161,472 measurements with surface thermometer17.
The laser technique added 3 to 10% of the procedure’s previous total value, presenting a reasonable cost versus benefit ratio.
The laser’s application in subcutaneous and deep dermis guided by the thermocamera allowed a more homogeneous energy application in the demarcated area and provided effective temperature monitoring. The surgeon obtained the desired effect without reaching the risk temperature for burns.
In this dynamic thermography article, the objective is not to evaluate an existing lesion or to measure recovery in the proposed technique. The aim was to avoid a possible internal deep burn injury, a proactive and preventive action through a thermoguided laser technique. However, considering the existence of dynamic, provocative tests in the evaluation of deep burns, this technique can be improved in future research accompanying patients submitted to this technique after the procedure to measure possible damage such as deep burns and better adjust the desired temperature with the laser.
CONCLUSION
Infrared skin thermography is a proper complementary method, non-invasive, without side effects, whose use during the lipolaser procedure provides reliable thermal monitoring, thus being an extra parameter that can avoid unwanted deep burns.
Lipolaser thermocamera monitoring also allowed a more homogeneous distribution of heat in the treated area. It provided the surgeon with safe monitoring to apply to the subcutaneous and deep dermis an adequate energy dose to obtain the desired effect.
Monitoring to the point of burning the skin with laser defines what temperature this technique offers risk and allows the surgeon to determine the target temperature he wants to reach in each body area.
Future longitudinal studies are suggested in which the potentials of cutaneous infrared thermography in the monitoring of the lipolaser process and its evaluation of the existence of deep subcutaneous burns with provocative thermal tests are suggested.
ACKNOWLEDGMENT
Thanks to Marcelo Kikuchi, from Laser Medical, for the support and supply of the equipment.
REFERENCES
1. Sociedade Brasileira de Cirurgia Plástica (SBCP). Censo 2016. Situação da Cirurgia Plástica no Brasil. Análise comparativa das pesquisas 2014 e 2016. São Paulo: SBCP; 2017; [acesso em 2019 Jun 15]. Disponível em: http://www2.cirurgiaplastica.org.br/wp-content/uploads/2017/12/CENSO-2017.pdf
2. Illouz YG. Body contouring by lipolisis: a 5-year experience with over 3000 cases. Plast Recons Surg. 1983 Nov;72(5):591-7.
3. Weiss RA, Beasley K. Laser assisted liposuction using a novel blend of lipid and water-selective wavelenghts. Lasers Surg Med. 2009 Dez;41(10):760-6.
4. Centurion P, Cuba JL, Noriega A. Lipossucción con diodo láser 980nm: optimización de protocolo seguro em cirurgía de contorno corporal. Cir Plást Iberolatinoam. 2011 Jun;37(4):355-64.
5. Dornelles RFV, Lima e Silva A, Missel J, Centurion P. Laserlipólise com diodo 980nm: experiência com 400 casos. Rev Bras Cir Plást. 2013;28(1):124-9.
6. Lawson R. Implications of surface temperatures in the diagnosis of breast cancer. Can Med Assoc J. 1956 Jun;75(4):309-10.
7. Chan LS, Lo JLF, Kumana CR, Cheung BMY. Utility of infrared thermography for screening febrile subjects. Hong Kong Med J. 2013 Apr;19(2):109-15.
8. Nahm FS. Infrared thermography in pain medicine. Korean J Pain. 2013 Jul;26(3):219-22.
9. Ang QY, Goh HJ, Cao Y, Li Y, Chan SP, Swain JL, et al. A new method of infrared thermography for quantification of brown adipose tissue activation in healthy adults: a randomized trial. J Physiol Sci. 2017 Mai;67(3):395-406.
10. Engel JM, Saier U. Thermographische standarduntersuchungen in der rheumatologie und richtlinien zu deren befundung. Baden-Baden: Verlag; 1984.
11. Moreira DG, Costello JT, Brito CJ, Adamczyk JG, Ammer K, Bach AJ, et al. Thermographic imaging in sports and exercise medicine: a Delphi study and consensus statement on the measurement of human skin temperature. J Thermal Biol. 2017 Out;69:155-62.
12. Herman C. The role of dynamic infrared imaging in melanoma diagnosis. Expert Rev Dermatol. 2013 Apr;8(2):177-84.
13. John HE, Niumsawatt V, Rozen WM, Whitaker IS. Clinical applications of dynamic infrared thermography in plastic surgery: a systematic review. Gland Surg. 2016 Abr;5(2):12-32.
14. Ley O, Deshpande C, Prapamcham B. Lumped parameter thermal model for the study of vascular reactivity in the fingertip. J Biomech Eng. 2008 Mai;130(3):031012.
15. Davey M, Eglin C, House J, Tipton M. The contribution of blood flow to the skin temperature responses during a cold sensitivity test. Eur J Appl Physiol. 2013 Set;113(9):2411-7.
16. Suzuki Y, Kobayashi M, Kuwabara K, Kawabe M, Kikuchi C, Fukuda M. Skin temperature responses to cold stress in patients with severe motor and intellectual disabilities. Brain Develop. 2013 Mar;35(3):265-9.
17. Key DJ. Integration of thermal imaging with surface radiofrequency thermistor heating. J Drugs Dermatol. 2014 Dez;13(12):1485-9.
18. Sadick N. Tissue tightening technologies. Aesthet Surg J. 2008 Mar;28(2):180-8.
19. Hayashi K, Thabit G, Massa KL, Bogdanske JJ, Cooley AJ, Orwin JF, et al. The effect of thermal heating on the length and histologic properties of the glenohumeral joint capsule. Am J Sports Med. 1997 Jan/Fev;25(1):107-12.
20. Pritzker RN, Hamilton HK, Dover JS. Comparison of different technologies for noninvasive skin tightening. J Cosmet Dermatol. 2014 Dez;13(4):315-23.
21. Lawrence JC, Bull JP. Thermal conditions which cause skin burns. Q IMechE. 1976;5(3):61-3.
22. Lawson RN, Gaston JP. Temperature measurements of localized pathological processes. Ann N Y Acad Sci. 1964 Out;121:90-8.
23. Mladick R, Goergiade N, Thorne F. A clinical evaluation of the use of thermography in determining degree of burn injury. Plast Reconstr Surg. 1966 Dez;38(6):512-8.
24. Watson AC, Vasilescu C. Thermography in plastic surgery. J R Coll Surg Edinb. 1972 Jun;17(4):247-52.
25. Anselmo V, Zawacki B. Infra-red photography as a diagnostic tool for the burn ward. Proc Soc Photo Opt Instr Eng. 1973;8:181.
26. Renkielska A, Nowakowski A, Kaczmarek M, Ruminksi J. Burn depths evaluation based on active dynamic IR thermal imaging--a preliminary study. Burns. 2006 Nov;32(7):867-75.
27. Prindeze NJ, Fathi P, Mino MJ, Mauskar NA, Travis TE, Paul DW, et al. Examination of the early diagnostic applicability of active dynamic thermography for burn wound depth assessment and concept analysis. J Burn Care Res. 2015 Nov/Dez;36(6):626-35.
28. Medina-Preciado JD, Kolosovas-Machuca ES, Velez-Gomez E, Miranda-Altamirano A, González FJ. Noninvasive determination of burn depth in children by digital infrared thermal imaging. J Biomed Optics. 2013;18(6):061204.
1. Hospital da Plástica SP, Department of Plastic
Surgery, São Paulo, SP, Brazil.
2. Kamamoto Clinic, Plastic Surgery, São Paulo,
SP, Brazil.
3. Poliscan Brasil, Medical equipment, São Paulo,
SP, Brazil.
FK Analysis and/or data interpretation, Conception and design study, Final manuscript approval, Realization of operations and/or trials, Supervision, Writing - Original Draft Preparation
OFN Analysis and/or data interpretation, Conception and design study, Data Curation, Methodology, Realization of operations and/or trials, Writing - Review & Editing
JOGR Conception and design study, Conceptualization, Investigation, Methodology, Project Administration, Realization of operations and/or trials, Supervision, Writing - Original Draft Preparation
CECS Analysis and/or data interpretation, Data Curation, Formal Analysis, Methodology, Software, Visualization TM Analysis and/or data interpretation, Conceptualization, Software, Writing - Review & Editing
Corresponding author: Fabio Kamamoto, Rua Mato Grosso, Higienópolis, 306, Conjunto 1402, São Paulo, SP, Brazil. Zip Code: 01239-040. E-mail: fabio.kamamoto@gmail.com
Article received: November 21, 2019.
Article accepted: October 22, 2020.
Conflicts of interest: none












 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter