

Original Article - Year 2022 - Volume 37 - Issue 3
FACE-Q Head and Neck Cancer Questionnaire for Brazilian Portuguese: Translation, Cross-Cultural Adaptation, and Linguistic Validation
Tradução, adaptação e validação linguística do FACE-Q Câncer de Cabeça e Pescoço para o português do Brasil
ABSTRACT
Introduction: Head and neck neoplasms often affect fundamental functions, such as swallowing, speech, eating, and socializing. Evaluating their treatment should consider the physician's opinion and the patient's perspective. This difficulty in assessing the success of treatment led to the development of the FACE-Q Head and Neck Cancer Module, a questionnaire of patient-reported outcomes that measure the appearance, facial function, quality of life, and experience of care to head and neck neoplasms. The objective is to translation, cultural adaptation, and linguistic validation of the FACE-Q Head and Neck Cancer questionnaire for Brazilian Portuguese.
Methods: The translation, cultural adaptation, and linguistic validation of the full questionnaire took place in four stages, using official guidelines from the World Health Organization and the International Society of Pharmacoeconomics and Outcomes Research.
Results: A semantic, idiomatic, and conceptually equivalent Brazilian Portuguese version was achieved through a linguistically validated translation of the English FACE-Q Head and Neck Cancer module.
Conclusion: The Brazilian Portuguese version presents a version equivalent to the original English instrument, which can be used as a critical patient-reported outcome assessment.
Keywords: Head and neck neoplasms; Reconstructive surgical procedures; Quality of life; Patient outcome assessment; Outcome assessment, health care.
RESUMO
Introdução: As neoplasias de cabeça e pescoço costumam afetar funções fundamentais, como engolir, falar, comer e se socializar. A avaliação do seu tratamento deve, portanto, levar em consideração a opinião do médico e a perspectiva do paciente. Essa dificuldade em avaliar o sucesso do tratamento levou ao desenvolvimento do FACE-Q - Módulo de Câncer de Cabeça e Pescoço, um questionário de resultados relatados pelo paciente que mede a aparência, função facial, qualidade de vida e experiência de cuidado para neoplasias de cabeça e pescoço. O objetivo é a tradução, adaptação cultural e validação linguística do questionário FACE- Q Câncer de Cabeça e Pescoço para o português brasileiro.
Métodos: A tradução, adaptação cultural e validação linguística do questionário completo ocorreram em quatro etapas, usando as diretrizes oficiais da Organização Mundial da Saúde e da Sociedade Internacional de Farmacoeconomia e Pesquisa de Resultados.
Resultados: Uma versão em português brasileiro semântica, idiomática e conceitualmente equivalente foi obtida por meio de uma tradução validada linguisticamente do módulo FACE-Q Head and Neck Cancer em inglês.
Conclusão: A versão em português brasileiro apresenta uma versão com equivalente ao instrumento original em inglês, que pode ser utilizada como avaliação crítica de resultados relatados pelo paciente.
Palavras-chave: Neoplasias de cabeça e pescoço; Procedimentos cirúrgicos reconstrutivos; Qualidade de vida; Avaliação de resultados da assistência ao paciente; Avaliação de resultados em cuidados de saúde.
INTRODUCTION
Head and neck neoplasms have a high incidence and represent significant morbidity and mortality, as they often affect fundamental functions, such as swallowing, speech, eating and socialization1, 2. The treatment of this pathology may involve multiple modalities, such as chemotherapy, radiotherapy and surgery, with varied involvement of these functions over time and often with the need for complex reconstruction processes3.
Therefore, evaluating the results of these procedures should consider the physician’s opinion and the patient’s perspective4. At the same time, evidence-based medicine has led to validated instruments that compare results from different authors.
This difficulty in assessing treatment success led to the development of the FACE-Q Head and Neck Cancer Module, a patient-reported outcome questionnaire (PRO - patient-reported outcome) that measures appearance, facial function, quality of life and experience of care for head and neck neoplasms5, 6, 7. The questionnaire divides the assessment into General Appearance of the Face, Eating and Drinking, Oral Competence, Salivation, Smiling, Talking, Swallowing, Appearance Distress, Cancer Concern, Drooling Distress, Food Distress, Smiling Distress, Talking Distress, and Information (Chart 1).
| Appearance | Facial Function | Quality of life | Care Experience |
|---|---|---|---|
| General Face | Eating and Drinking | Appearance Affliction | Information |
| Oral Competence | Cancer Concern | ||
| Salivation | Worry about drooling | ||
| Smile | Affliction to eat | ||
| Speak | Affliction to smile | ||
| Swallow | Affliction to speak |
Due to linguistic and cultural differences between the English-speaking population of the FACE-Q Head and Neck Cancer Module and the Brazilian population, translation, cultural adaptation, and linguistic validation of the questionnaire are essential to evaluate patients undergoing treatment for head and neck cancer in Brazil.
OBJECTIVE
The aim of this study was the translation, cultural adaptation and linguistic validation of the FACE-Q Head and Neck Cancer questionnaire into Brazilian Portuguese.
METHODS
This study was approved and authorized by the Q-Portfolio team and the association that manages its academic and commercial distribution.
Participants were selected by convenience at the Cancer Institute of the State of São Paulo (ICESP) under approval by the Research Ethics Committee (CAE 34797120.6.0000.0068) and provided written informed consent. This study followed all procedures following the ethical standards of the institutional review board and the 1964 Declaration of Helsinki and its later amendments.
FACE-Q Head and Neck Cancer: The Questionnaire
The FACE-Q Head and Neck Cancer is a PRO (patient-reported outcome) questionnaire applied in the clinical or research environment to collect patient responses directly. It quantifies aspects of quality of life and outcome variables from the patient’s perspective, such as satisfaction, symptoms and adverse effects. PRO instruments are a way of quantifying how patients perceive their health and the impact of procedures on their quality of life8.
The entire instrument consists of 164 items, divided into 14 scales within four conceptual frameworks: Appearance, Facial Function, Quality of Life and Care Experience (Chart 1).
Stages of translation, cross-cultural adaptation and linguistic validation
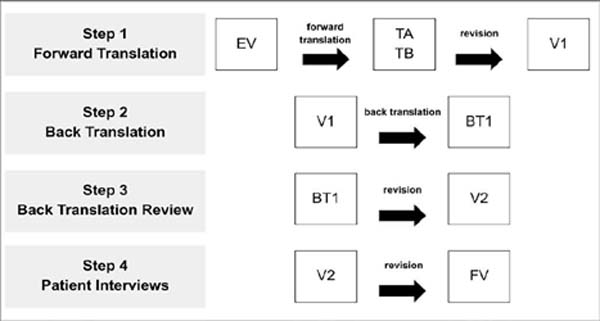
The translation, cultural adaptation and linguistic validation of the complete questionnaire took place in four stages (Figure 1) from July 2020 to September 2020, following recommended practices9, 10. After each step, a report was produced, highlighting the process, the difficulties encountered, and the solutions implemented.
The focus of the authors, as well as the translators, was to maintain equivalence9:
Semantic equivalence: check if the translated words have the same meaning.
Idiom equivalence: formulate equivalent expressions in the target version, avoiding difficulties related to the translation of colloquialisms and idiomatic expressions.
Empirical equivalence: replacing questionnaire words with other similar terms used in our home culture.
Conceptual equivalence: observing whether words have different meanings across cultures, replacing inappropriate terms.
Step 1: Direct Translation
The questionnaire was translated from English into Brazilian Portuguese by two independent translators, native speakers of Portuguese and fluent in English; both had experience in healthcare translation.
Translators were instructed to translate using consistent and straightforward terminology rather than literal translations and were encouraged to provide feedback on their difficulties. At the end of the process, two versions were produced: translation A (TA) and translation B (TB)10.
The two translations were analyzed by three of the project’s authors and reconciled into a consensus version in Portuguese (V1) based on elements of the two initial versions.
Step 2: Reverse Translation
In the back-translation stage, V1 was translated from Portuguese back into English by a third native American English translator, fluent in Brazilian Portuguese and with experience in the healthcare field. This process generated the reverse translation (BT) version.
Step 3: Review the Reverse Translation
The authors and one of the developers of the original questionnaire compared the original English version (EV) with BT to identify possible semantic differences between the two versions. This step produced some changes in V1 and generated a second version in Portuguese (V2).
Step 4: Interviews with Patients
Version V2 was used for cognitive interviewing and debriefing in ten patients (convenience sample) undergoing surgical treatment for head and neck cancer and native speakers of Brazilian Portuguese:
Cognitive interview: individual interviews were conducted to assess the patient’s understanding of the questionnaire and confirm their interpretation of each question.
Debriefing: During the interview, each item in which the patient expressed doubts about the question or the answer was reviewed. These items were noted as well as the patients’ suggestions for more understandable terms within the context of the question.
The authors evaluated all the items that generated doubts among the patients and their suggestions. All changes were reviewed for wording and agreement by a native Portuguese-speaking editor with proficiency in English to eliminate any spelling errors and align possible errors in the agreement. The authors reviewed the adjustments and produced the final version (VF).
RESULTS
Two professional translators with knowledge in the health area performed the first stage of the translation process (Direct Translation). No difficulties in understanding the original questionnaire were reported during the process. The TA and TB versions were reconciled, preserving semantic, idiomatic, empirical and conceptual equivalence. This process showed a discrepancy in 80 of the 164 items (48.8%) in the original questionnaire (Table 1). AT was maintained on 72 occasions (90%) in the conciliation process between the versions, while TB was maintained on five (6.3%). An intermediate solution was necessary on three occasions (3.8%). After this reconciliation between the TA and TB versions, V1 was defined. In this version, colloquial language was used that was easy to understand by the target audience.
| Scales | Total number of items | Total different items | Maintained TA | Maintained TB | New/mixed solution |
|---|---|---|---|---|---|
| General Face | 15 | 6 | 4 | 2 | 0 |
| Eat and drink | 12 | 4 | 4 | 0 | 0 |
| Oral Competence | 9 | 5 | 5 | 0 | 0 |
| Salivation | 12 | 7 | 7 | 0 | 0 |
| Smile | 11 | 3 | 2 | 0 | 1 |
| Speak | 11 | 5 | 4 | 0 | 1 |
| Deglutition | 12 | 6 | 4 | 2 | 0 |
| Affliction with appearance | 11 | 3 | 3 | 0 | 0 |
| Affliction to eat | 11 | 9 | 9 | 0 | 0 |
| Drooling affliction | 10 | 8 | 8 | 0 | 0 |
| Affliction to smile | 9 | 3 | 3 | 0 | 0 |
| Affliction to speak | 13 | 8 | 8 | 0 | 0 |
| Cancer concern | 13 | 7 | 7 | 0 | 0 |
| Information | 15 | 6 | 4 | 1 | 1 |
| Total (%) | 164 (100%) | 80 (48.8%) | 72 (90%) | 5 (6.3%) | 3 (3.8%) |
As an example of a discrepancy, in the translation of the original item “Eu tenho dificuldades para me alimentar por causa da minha boca seca”, both TA (“Eu tenho dificuldades para me alimentar por causa da minha boca seca.”) and TB (“Tenho problemas para comer devido a minha boca seca.”) were created. In this case, AT was chosen due to the greater ease of understanding. In another item (“I have a problem being understood when speaking face-to-face.”) the TA version (“Eu tenho problemas para ser compreendido quando falo cara a cara “) and TB (“Tenho problemas para ser entendido quando falo cara a cara “) were merged to form the final option (“Eu tenho problemas para ser compreendido/entendido quando falo cara a cara.”)
In the second stage of the translation process (Reverse Translation), version V1 was back-translated into English by another professional translator whose native language was English, giving rise to the BT version.
In the third step (Reverse Translation Review), the Q-Portfolio team reviewed the BT version to identify errors in comprehension, translation or lack of precision concerning the original version (EV). Among the 164 items present in the questionnaire, 56 (34.1%) showed exact correspondence with the original version (EV), while 108 (65.9%) showed small textual variations (Table 2). However, all these items kept their original meaning, and revisions were unnecessary. Therefore, version V1 was left unchanged at this stage, producing version V2.
| Scales | Total number of items | Total of different items | Need for review |
|---|---|---|---|
| General Face | 15 | 8 | 0 |
| Eat and drink | 12 | 10 | 0 |
| Oral Competence | 9 | 3 | 0 |
| Salivation | 12 | 7 | 0 |
| Smile | 11 | 5 | 0 |
| Speak | 11 | 9 | 0 |
| Deglutition | 12 | 4 | 0 |
| Appearance Affliction | 11 | 9 | 0 |
| Affliction to eat | 11 | 10 | 0 |
| Drooling affliction | 10 | 6 | 0 |
| Affliction to smile | 9 | 6 | 0 |
| Affliction to speak | 13 | 10 | 0 |
| Cancer concern | 13 | 10 | 0 |
| Information | 15 | 11 | 0 |
| Total (%) | 164 (100%) | 108 (65.9%) | 0 |
As an example of the differences found between the EV and BT versions, the item “I have a problem drinking from a cup.” (EV) originated from the version “ Tenho problemas para beber de um copo. “ (BT), and also the item “I cannot communicate emotions with my smile.” (EV), which gave rise to the version “ Não consigo demonstrar emoções com meu sorriso. “ (BT).
Finally, in the fourth stage (Patient Interviews), individual interviews were carried out with ten patients who underwent head and neck cancer treatment to assess possible difficulties in understanding and confirming the correct interpretation of all items. Four patients were female, and six were male. The age range of these patients ranged from 57 to 87 years, with a median of 69 years. As for the histological type, seven patients had squamous cell carcinoma, and the others had mucoepidermoid carcinoma, basal cell carcinoma and osteosarcoma. Finally, regarding the location of the tumors, the affected regions were the forehead, orbit, maxilla (2), the mandible (2), tongue (2), lower lip and salivary gland. All questions, alternatives and suggestions resulting from this process were evaluated. Among the 164 items, , 21 alterations were suggested by the patients (12.8%) to facilitate the understanding of the questionnaire items (Table 3).
| Scales | Total number of items | Need for review |
|---|---|---|
| General Face | 15 | 2 |
| Eat and drink | 12 | 6 |
| Oral Competence | 9 | 1 |
| Salivation | 12 | 2 |
| Smile | 11 | 2 |
| Speak | 11 | 2 |
| Deglutition | 12 | 1 |
| Affliction with Appearance | 11 | 0 |
| Affliction to eat | 11 | 0 |
| Drooling affliction | 10 | 0 |
| Affliction for Smiling | 9 | 0 |
| Affliction to speak | 13 | 3 |
| Cancer concern | 13 | 0 |
| Information | 15 | 2 |
| Total (%) | 164 (100%) | 21 (12.8%) |
As an example of this last step, the original item “My face looks unattractive” originated the version “ Meu rosto parece pouco atraente. “ (V2), which, after the interview with the patients, was changed to “Meu rosto não parece atraente,” due to the greater adequacy of the terms used. In another example, the original version “I get frustrated when I speak” gave rise to the version “Eu me sinto frustrado quando falo”. (V2), which, after the interview with the patients, was changed to “Eu me sinto frustrado/chateado quando falo” due to the difficulty in understanding the word “frustrado.”
A final version (VF) was produced in Brazilian Portuguese, which preserved equivalent concepts and was easy to understand for the target population. This version can be obtained for academic purposes free of charge11.
DISCUSSION
The FACE-Q Head and Neck Cancer questionnaire was designed to assess satisfaction with specific functional, aesthetic and psychological aspects of head and neck cancer5, 6, 7. After translation, cultural adaptation and linguistic validation, the questionnaire must be applied in its original language or in other languages. So far, the questionnaire is only available in English.
Our process used the same methodology as other studies that translated the FACE-Q12, 13, 14, 15, 16, focusing on a translation that maintains the original semantics and idea of the questions, avoiding literal translation to facilitate patient understanding. Using official guidelines from the World Health Organization (WHO) and the International Society for Pharmacoeconomics and Outcome Research (ISPOR), we obtained a linguistically validated translation of the English module FACE-Q Head and Neck Cancer into a semantic, idiomatic and conceptually equivalent version. in Brazilian Portuguese10, 17, 18.
Experienced translators in the health area proved essential, as they often use different terms than non-specialists17. Furthermore, the interview with the patients (Step 4) proved useful in our case, as the patient feedback was broad and led to critical linguistic changes.
Although the translation is primarily accurate concerning the original questionnaire, a literal translation is never possible, so there is always a slight interpretation bias.
CONCLUSION
After the process of translation, cultural adaptation and linguistic validation, the FACE-Q Head and Neck Cancer questionnaire in the Brazilian Portuguese version presents a version equivalent to the original instrument in English, which can be used as an acritical evaluation of patient-reported outcomes (PRO), both in research and in clinical practice.
REFERENCES
1. Gliklich RE, Goldsmith TA, Funk GF. Are head and neck specific quality of life measures necessary? Head Neck. 1997;19(6):474-80. DOI: 10.1002/(sici)1097-0347(199709)19:6<474::aid-hed3>3.0.co;2-w
2. Funk GF, Karnell LH, Christensen AJ. Long-term health-related quality of life in survivors of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2012;138(2):123-33. DOI: 10.1001/archoto.2011.234.
3. El-Deiry M, Funk GF, Nalwa S, Karnell LH, Smith RB, Buatti JM, et al. Long-term quality of life for surgical and nonsurgical treatment of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2005;131(10):879-85. DOI: 10.1001/archotol.131.10.879
4. Chera BS, Eisbruch A, Murphy BA, Ridge JA, Gavin P, Reeve BB, et al. Recommended patient-reported core set of symptoms to measure in head and neck cancer treatment trials. J Natl Cancer Inst. 2014;106(7):dju127. DOI: 10.1093/jnci/dju127
5. Cracchiolo JR, Klassen A F, Young-Afat DA, Albornoz CR, Cano SJ, Patel SG, et al. Leveraging patient-reported outcomes data to inform oncology clinical decision making: Introducing the FACE-Q Head and Neck Cancer Module. Cancer. 2019;125(6):863-72. DOI: 10.1002/cncr.31900
6. Albornoz CR, Pusic AL, Reavey P, Scott AM, Klassen A F, Cano SJ, et al. Measuring health-related quality of life outcomes in head and neck reconstruction. Clin Plast Surg. 2013;40(2):341-9. DOI:10.1016/j.cps.2012.10.008
7. Pusic A, Liu JC, Chen CM, Cano S, Davidge K, Klassen A, et al. A systematic review of patient-reported outcome measures in head and neck cancer surgery. Otolaryngol Head Neck Surg. 2007;136(4):525-35. DOI: 10.1016/j.otohns.2006.12.006
8. Cano SJ, Klassen A, Pusic AL. The science behind quality-of-life measurement: a primer for plastic surgeons. Plast Reconstr Surg. 2009;123(3):98e-106e. DOI: 10.1097/PRS.0b013e31819565c1
9. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-91. DOI: 10.1097/00007632-200012150-00014
10. Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al.; ISPOR Task Force for Translation and Cultural Adaptation. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8(2):94-104. DOI: 10.1111/j.1524-4733.2005.04054.x
11. Q-Portfolio. Setting the bar in patient reported outcome measurement. Disponível em: http://qportfolio.org/
12. Ottenhof MJ, Lardinois AJPM, Brouwer P, Lee EH, Deibel DS, van der Hulst RRWJ, et al. Patient-reported Outcome Measures: The FACE-Q Skin Cancer Module: The Dutch Translation and Linguistic Validation. Plast Reconstr Surg Glob Open. 2019;7(10):e2325. DOI: 10.1097/GOX.0000000000002325
13. Tan SK, Leung WK, Tang ATH, Tse ECM, Zwahlen RA. Orthognathic Relevant Scales of FACE-Q: Translation and Validation for Hong Kong Chinese Patients. Plast Reconstr Surg Glob Open. 2017;5(12):e1608. DOI: 10.1097/GOX.0000000000001608
14. Cogliandro A, Barone M, Persichetti P. Italian Linguistic Validation of the FACE-Q Instrument. JAMA Facial Plast Surg. 2017;19(4):336-7. DOI: 10.1001ZjamafaciaL2016.2103
15. Radulesco T, Mancini J, Penicaud M, Dessi P, Michel J. Assessing normal values for the FACE-Q rhinoplasty module: An observational study. Clin Otolaryngol. 2018;43(4):1025-30. DOI: 10.1111/coa.13086
16. Poulsen L, Rose M, Klassen A, Roessler KK, Sørensen JA. Danish translation and linguistic validation of the BODY-Q: a description of the process. Eur J Plast Surg. 2017;40(1):29-38. DOI: 10.1007/s00238-016-1247-x
17. World Health Organization (WHO). Process of translation and adaptation of instruments. Geneva: WHO; 2017.
18. Acquadro C, Conway K, Girourdet C, Mear I. Linguistic validation manual for Patient-Reported Outcomes (PRO) instruments, By C. Acquadro, K. Conway, C. Girourdet & I. Mear, MAPI ResearchTrust, Lyon, France, 2004,184 pp, ISBN: 2-9522021-0-9, price €70/$90. Qual Life Res. 2005;14(7):1791-2. DOI: 10.1007/s11136-005-5367-1
1. Universidade de São Paulo, São Paulo, SP, Brazil.
2. Instituto do Câncer do Estado de São Paulo, São Paulo, SP, Brazil.
VPFP Analysis and/or interpretation of data, Conception and design of the study, Writing - Preparation of the original
RCL Statistical Analysis, Data Collection, Conception and Design of the Study
AMBB Statistical Analysis, Data Collection, Conception and Design of the Study
CPL Statistical Analysis, Data Collection, Research
RMCT Methodology, Supervision, Validation
FFB Final Manuscript Approval, Project Management, Supervision
RG Final Manuscript Approval, Resource Management, Project Management
Corresponding author: Vitor Penteado Figueiredo Pagotto Rua Ovídio Pires de Campos, nº 225 5º andar - Cerqueira Cesar, São Paulo, SP, Brazil Zip Code 05403-010 E-mail: vitorpfpagotto@gmail.com
Article received: May 24, 2021.
Article accepted: July 14, 2021.
Conflicts of interest: none.
Institution: Universidade de São Paulo, Faculdade de Medicina, Divisão de Cirurgia Plástica, São Paulo, SP, Brazil.








 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter