

Original Article - Year 2025 - Volume 40Issue 1
Evaluation of Asymptomatic Rupture of Breast Implants Filled with Silimed Silicone Gel
Avaliação da ruptura assintomática de implantes mamários preenchidos com gel de silicone Silimed
ABSTRACT
Introduction: Breast augmentation is a cosmetic surgery dating back to 1895, which gained popularity in the 1960s with the introduction of silicone implants. Although it is a common procedure, complications such as implant rupture can occur. "Silent" rupture is particularly concerning, as it presents no symptoms and requires careful monitoring. The United States Food and Drug Administration (FDA) recommends ultrasound (USG) or magnetic resonance imaging (MRI) to identify silent rupture.
Objective: To evaluate the rate of asymptomatic rupture in patients with smooth and textured external envelope breast implants from the Silimed (Silimed Indústria de Implantes Ltda.) brand through MRI.
Materials and Methods: We selected 97 surgeons from different cities in Brazil who kept records of patients with Silimed. Patients with implants from other manufacturers, pregnant women, and those with contraindications for MRI were excluded. The selected patients underwent clinical exams and MRI scans, with image analysis performed by independent radiologists. Suspected ruptures were confirmed surgically.
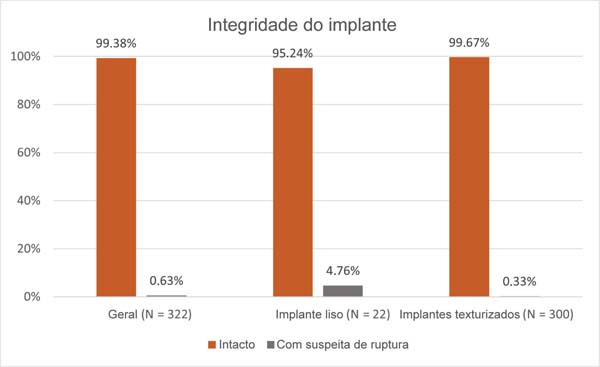
Results: Of the 161 patients evaluated, only 2 (0.63%) had a suspected implant rupture confirmed surgically, both after 9 years of implantation: one with a smooth implant and the other with a textured implant. Most Silimed implants were considered intact: 99.67% of the textured and 95.24% of the smooth implants.
Conclusion: The present study contributes to scientific knowledge about breast implant ruptures and suggests that Silimed implants are s
RESUMO
Introdução: O aumento mamário é uma cirurgia estética praticada desde 1895, e que ganhou popularidade na década de 1960 com o surgimento dos implantes de silicone. Embora seja um procedimento comum, podem ocorrer complicações, como a ruptura dos implantes. A ruptura "silenciosa" é especialmente preocupante, pois não apresenta sintomas e requer monitoramento cuidadoso. A United States Food and Drug Administration (FDA) indica os exames de ultrassonografia (USG) ou ressonância magnética (RM) para identificar a ruptura silenciosa.
Objetivo Materiais e Métodos: Foram selecionados 97 cirurgiões de diversas cidades brasileiras com prontuários de pacientes que utilizavam implantes Silimed. Foram excluídas pacientes com implantes de outros fabricantes, gestantes, e aquelas com contraindicações para a RM. As pacientes selecionadas realizaram exames clínicos e RM, e as imagens foram analisadas por radiologistas independentes. Suspeitas de ruptura foram confirmadas cirurgicamente.
Resultados: Entre as 161 pacientes avaliadas, 2 (0,63%) apresentaram suspeita de ruptura confirmada cirurgicamente, ambas com 9 anos de implantação: uma com implante liso e outra, com texturizado. A maioria dos implantes foi considerada íntegra: 99,67% dos texturizados e 95,24% dos lisos.
Conclusão: Este estudo contribui para o conhecimento científico sobre rupturas de implantes mamários, e sugere que os implantes Silimed são seguros. A taxa de ruptura assintomática em implantes com 5 a 10 anos de implantação foi baixa.
Palavras-chave: eventos adversos; gel de silicone; implantes mamários; ruptura assintomática; ruptura do implante
Introdução
A história do aumento mamário remonta a 1895, quando foi realizado o primeiro procedimento deste tipo, documentado pelo cirurgião alemão Vincenz Czerny. Ele fez uso de tecido adiposo autólogo para reconstruir uma mama que havia sido removida devido a um lipoma benigno.1 Esse foi o marco do início das técnicas cirúrgicas destinadas a melhorar o tamanho e a forma dos seios. A partir de então, muitos pioneiros desenvolveram novas técnicas cirúrgicas e contribuíram significativamente para o campo do aumento mamário.
Thomas Cronin e Frank Gerow receberam destaque por terem sido os primeiros a utilizar implantes preenchidos com gel de silicone em 1962.2,3 Desse momento em diante, outros cirurgiões inspirados começaram a publicar artigos sobre suas técnicas e também contribuíram relevantemente para o aperfeiçoamento da cirurgia de aumento mamário,4 como foram os casos de Dempsey e Latham,5 Griffiths,6 Regnault,7 Hoehler,8,9 Eiseman,10 Ho11 e Price et al.12 Após todos esses avanços, os fabricantes de implantes se empenharam para implementar melhorias em seus dispositivos, com o intuito de reduzir possíveis eventos adversos e promover aumento da satisfação e qualidade de vida de mulheres submetidas à cirurgia mamária.13
Os implantes mamários evoluíram ao longo do tempo para melhorar sua segurança e os resultados estéticos.3,14 A primeira geração, nos anos 1960, continha gel viscoso e apresentava altas taxas de contratura capsular; na segunda geração, reduziu-se a espessura da membrana, mas isso aumentou as taxas de ruptura e migração do silicone.15 Na terceira geração, foram introduzidos invólucros reforçados e barreiras de difusão, ao passo que, na quarta, melhorou-se a coesividade do gel.3,14 Por fim, a quinta geração contém gel altamente coesivo e apresenta formatos anatômicos, o que resulta em maior estabilidade e segurança.15
De acordo com a pesquisa internacional da International Society of Aesthetic Plastic Surgery (ISAPS) sobre procedimentos estéticos/cosméticos realizada em 2023,16 o aumento mamário é o segundo procedimento mais realizado no mundo (1.892.777 procedimentos). No entanto, a utilização de implante mamário pode ocasionar algumas complicações, e as mais frequentes relacionadas ao implante mamário incluem contratura capsular e ruptura do implante.4
A ruptura da membrana do implante pode ocorrer por lesão traumática ou até mesmo por desgaste natural ao longo do tempo.17 A ruptura pode ser classificada como extra ou intracapsular. A extracapsular se caracteriza quando o gel de silicone se estende para fora da cápsula fibrosa, o que pode causar inchaço localizado, endurecimento da mama, dor, migração do gel e possível deformidade mamária. Em contrapartida, a ruptura intracapsular ocorre quando o gel de silicone vaza do implante e se acumula dentro da cápsula fibrosa que se forma naturalmente em volta do implante. Nesse caso, os sintomas podem ser leves ou inexistentes, e podem incluir um leve endurecimento da mama, alteração na forma ou no tamanho da mama e dor leve.17 As rupturas intracapsulares são mais comuns e mais difíceis de diagnosticar clinicamente por normalmente serem assintomáticas, sendo denominadas rupturas silenciosas.18,19
Apesar de haver um debate acerca da interpretação correta da ressonância magnética (RM), a qual, se não realizada com cautela, leva a resultados falsos-positivos ou negativos,20,21 ela ainda é considerada o método mais acurado para avaliar a integridade dos implantes mamários.18-22 Apresenta alta sensibilidade para detectar rupturas de implantes mamários, superando significativamente a sensibilidade da mamografia.20,22 A confirmação da ruptura ocorre no momento da cirurgia, aliada ao laudo dos exames de imagem. Em 2020, a United States Food and Drug Administration (FDA publicou o documento “Breast Implants - Certain Labeling Recommendations to Improve Patient Communication”,23 no qual aconselha que a paciente realize o primeiro ultrassom ou RM 5 a 6 anos após a data da implantação, mesmo que não haja sintomas de ruptura. Após esse primeiro exame de imagem, a recomendação é realizá-lo novamente a cada 2 a 3 anos. Entretanto, se houver qualquer sintoma anormal ou algum exame de imagem com laudo inconclusivo antes desse prazo, recomenda-se realizar a RM assim que possível.23
Devido à importância e à relevância do monitoramento da ruptura silenciosa, realizamos um estudo descritivo com o objetivo de avaliar as taxas de ruptura assintomática em pacientes com implantes mamários Silimed (Silimed Indústria de Implantes Ltda.) por meio de RM e com confirmação por cirurgia.
Materiais e Métodos
Este estudo foi totalmente patrocinado pela Silimed, e todas as participantes assinaram um termo de consentimento. Na ocasião em que o estudo foi conduzido, a autora, professora do curso de pós-graduação em Cirurgia Plástica da Pontifícia Universidade Católica do Rio de Janeiro, integrado à 38ª enfermaria da Santa Casa de Misericórdia do Rio de Janeiro, onde funcionava o Serviço de Cirurgia Plástica do Professor Pitanguy. A autora foi patrocinada pela Silimed para conduzir este estudo como investigadora principal, e os exames de RM das pacientes solicitados pela médica também foram patrocinados pela empresa. Os dados foram coletados e analisados segundo rigorosos padrões de ética profissional.
Este estudo foi realizado por exigência da FDA, e teve o objetivo de atualizar os dados de segurança dos implantes preenchidos com gel de silicone e com superfícies lisas e texturizadas da marca Silimed de forma ambispectiva. Na primeira parte do estudo retrospectivo, 97 cirurgiões de diferentes cidades do Brasil foram selecionados. Os critérios de inclusão foram cirurgiões que realizaram cirurgia mamária utilizando implantes de silicone Silimed entre 1990 e 2000, e que possuíam prontuários organizados das pacientes. Como o intuito do estudo não era fazer uma comparação entre diferentes marcas, embora os médicos tivessem utilizado outras marcas de implantes, somente pacientes portadoras de implantes Silimed foram incluídas. A segunda parte consistiu em selecionar, por meio de busca nos prontuários do consultório desses cirurgiões, mulheres portadoras dos implantes Silimed. A parte prospectiva do estudo, que consistiu na avaliação clínica, exame e conduta médica, ocorreu entre 2006 e 2010, e foi realizada no Hospital PróCardíaco, na cidade do Rio de Janeiro. Ele foi aprovado pelo Comitê de Ética em Pesquisa (CEP) do Centro de Ensino e Pesquisas do Pró-Cardíaco (PROCEP; registro no CEP: 174). Por definição da empresa patrocinadora do estudo, foram excluídas pacientes portadoras de implantes metálicos, gestantes, aquelas com tatuagens e/ou qualquer outra contraindicação para RM, além de pacientes com implantes de outros fabricantes. Além disso, foram desconsideradas pacientes com mais de 10 anos de implantação devido à recomendação de vida útil contida na instrução de uso do produto.
Foram analisados implantes redondos e anatômicos, com proporção predominante de redondos. É importante pontuar que não houve avaliação de implantes revestidos com espuma de poliuretano, uma vez que, como mencionado anteriormente, este estudo foi uma exigência da FDA, situada nos Estados Unidos, onde esses tipos de implantes não são comercializados.
As pacientes incluídas possuíam implantes mamários Silimed com diversos tempos de implantação, e foram submetidas a exame clínico e RM. Vale ressaltar que apesar, de a FDA indicar exames de imagem a partir dos 5 anos de implantação, as participantes foram selecionadas consecutivamente para a realização dos exames, independente do período de implantação.
Todos os es exames clínicos foram realizados pela autora deste estudo. Os exames de RM foram realizados em seis centros médicos, dependendo do local de residência do paciente. Em todos os centros, as imagens foram obtidas utilizando um aparelho da General Electric de 1,5 T com bobinas mamárias dedicadas, seguindo o mesmo protocolo. As imagens de RM foram enviadas para três radiologistas, sendo um o radiologista do estudo e os outros dois, consultores, de forma que a identificação fosse apenas um número de controle. A avaliação ocorreu individualmente por meio de formulários padronizados. Para garantir imparcialidade, a análise foi feita de forma cega, sem acesso a qualquer informação dos pacientes, incluindo relatos anteriores. Os implantes foram considerados com suspeita de ruptura quando pelo menos dois especialistas concordaram. Todas as pacientes com indicativo de ruptura pela RM foram indicadas para cirurgia. A ruptura foi confirmada no momento da cirurgia nos casos em que houve descontinuidade do invólucro do implante, com ou sem vazamento do gel. Foi realizada análise estatística descritiva com variáveis categóricas e quantitativas.
Resultados
De um total de 404 pacientes recrutadas, 243 foram excluídas por possuírem implantes diferentes dos dispositivos em estudo, por estarem fora do período do estudo, ou por qualquer outro motivo que atendesse aos critérios de exclusão. Assim, 161 pacientes (322 implantes; 22 lisos e 300 texturizados) foram submetidas a exame clínico e RM. A idade média delas era de 44,26 anos, e o tempo médio de implantação, de 8,04 anos.
O tempo máximo de implantação das participantes era de 10 anos. Das pacientes examinadas, 2 (0,63%) tiveram o diagnóstico de suspeita de ruptura, 1 com implante liso e 1 com implante texturizado, ambas com 9 anos de implantação. Por meio da RM, identificamos que 99,67% dos implantes texturizados estavam íntegros, assim como 95,24% dos implantes lisos (►Fig. 1).
Discussão
Neste estudo, a taxa de ruptura foi de 0.63% (2 implantes) em 10 anos. Hölmich et al.24 (2003) realizaram um estudo utilizando 3 gerações de implantes mamários preenchidos com gel de silicone ao longo de 2 anos. Ao todo, 317 implantes provenientes de 271 pacientes que realizaram cirurgia com implante mamário entre os anos de 1973 a 1998 foram avaliados. Foi observado taxa de ruptura confirmada de 10% (33 implantes) e 7% de possíveis rupturas (23 casos sem confirmação). Através de uma curva estimada de ruptura, eles estabeleceram que no período de 3 a 10 anos é possível ter uma taxa de 15% de rupturas.24 Pitanguy et al.25 colaboradores (2010) realizaram uma avaliação de 5 anos na Clínica Ivo Pitanguy entre os anos de 2004 e 2009. Eles avaliaram 59 pacientes (129 mamas) e apesar de não terem explicitado uma taxa de ruptura, houve somente 2 procedimentos cirúrgicos devido à ocorrência de ruptura ao longo do estudo.
Scaranelo et al.26 (2004) avaliaram 83 implantes com suspeita de ruptura silenciosa provenientes de 44 pacientes. Desses, 30 implantes (39%) estavam rompidos no momento da cirurgia, e 53 (64%) não apresentavam ruptura. Além disso, 27 desses implantes (32,5%) sem ruptura apresentavam vazamento de gel e 26 (31,5%) estavam intactos. A idade média dos implantes rompidos foi de 11,9 anos,26 o que corrobora os achados que indicam que, quanto maior o tempo de implantação, maiores as chances de ruptura. Em 2018, Stevens et al.27 realizaram o Sientra Core Study, um estudo de 10 anos, aberto, prospectivo e multicêntrico, projetado para avaliar a segurança e a eficácia de implantes mamários da Sientra (Tiger Aesthetics Medical, LLC). Nessa época, a marca americana era representante autorizada e distribuidora dos implantes Silimed nos Estados Unidos. Os autores27 realizaram exames de RM em 571 pacientes a partir do terceiro ano de implantação, e observaram um risco de ruptura por meio da estimativa de Kaplan-Meier de 8,6% (grupos aumento, revisão de aumento, reconstrução e revisão de reconstrução). É importante ressaltar que existem particularidades em cada estudo publicado,28,29 como o tamanho amostral, o tempo de acompanhamento, o delineamento estatístico e o desenho experimental, que dificultam uma comparação direta e objetiva dos resultados. Assim, as diferenças nas taxas devem ser interpretadas com cautela, levando-se em consideração as variações no rigor metodológico dos estudos publicados que influenciam nos resultados.
A mamografia, a ultrassonografia e a RM são os exames de imagem mais frequentemente usados para avaliar a integridade dos implantes mamários, geralmente em pacientes sintomáticas de ruptura. Embora avanços nas técnicas de ultrassom estejam surgindo como alternativas potenciais,30 a RM ainda é considerada o exame mais preciso e sensível para a detecção da integridade dos implantes.20,21,31-33
Neste estudo, das duas rupturas encontradas, uma ocorreu em implante liso e outra, em implante texturizado. Embora na literatura alguns eventos adversos, como a contratura capsular, sejam mais associados a uma determinada superfície, no que tange à ruptura, a literatura não apresenta um consenso. As taxas de ruptura dos implantes mamários texturizados variam entre os estudos, com uma taxa média de 3% a 15,1%, e são influenciadas por fatores como tipo de implante e duração do uso. Haws et al.34 (2015) relataram baixa prevalência de ruptura nos implantes texturizados quando comparados com implantes lisos (de 0,8% e 3,8%, respectivamente).34 Os casos de ruptura aqui apresentados ocorreram em pacientes implantadas havia 9 anos. Apesar de não ter como afirmar que a ruptura de fato ocorreu no nono ano, devido ao seu caráter “silencioso”, os dados corroboram a unanimidade dos achados da literatura35 de que os casos de ruptura estão associados ao maior tempo de implantação.
As altas taxas de implantes mamários íntegros, de 99,67% para os texturizados e de 95,24% para os lisos, indicam um desempenho robusto, que se alinha aos achados36 de que a coesividade do gel dos implantes mamários é um ponto relevante. Esses dispositivos são produzidos com gel coesivo de alto desempenho (High-Strength Cohesive-Plus, HSC þ , Sientra), com maior índice de integração do gel com a membrana, e apresentam o índice máximo de elasticidade do gel e maior estabilidade do formato.37 São dispositivos pensados e projetados para serem resistentes a possíveis danos ocasionados pelas técnicas cirúrgicas e as singularidades das pacientes. Isso demonstra a preocupação das indústrias na melhoria constante das matérias-primas que compõem os implantes, preocupação esta que se estende até os dias atuais. A longevidade desses dispositivos torna cada vez mais relevante a avaliação de produtos com longos períodos de implantação a fim de demonstrar sua segurança e desempenho.
A ruptura do implante é geralmente tardia, muitas vezes, silenciosa, é de difícil diagnóstico clínico sem o auxílio de exames de imagem, além de apresentar diversas etiologias.18,34 Alguns autores relataram que, em mulheres com implantes mamários preenchidos com gel de silicone, 8% das rupturas são assintomáticas, e cerca de 33%, sintomáticas. Quando sintomáticas, essas mulheres apresentam dor, contratura capsular, alteração da forma do implante e assimetria mamária.38 O fato de uma parte das mulheres com implantes rompidos serem assintomáticas e a possibilidade de uma ruptura evoluir com vazamento de silicone para o corpo reforçam a indicação de monitoramento dos implantes mamários por meio de exames de imagens. Apesar da preocupação com essa complicação, os dados sobre ruptura silenciosa são ainda desconhecidos, muito provavelmente devido à falta de triagem e de relatórios padronizados.39,40
Os resultados indicam que, embora as rupturas sejam raras, a necessidade de monitoramento regular permanece crítica, pois casos assintomáticos ainda podem representar riscos à saúde.41 Os resultados deste estudo podem estimular novas pesquisas sobre o desempenho em longo prazo de diferentes tipos de implantes, particularmente em relação à sua coesividade e idade.
Conclusão
Com base nos dados aqui apresentados, conclui-se que a taxa de rupturas assintomáticas em pacientes com implantes mamários lisos e texturizados da Silimed, avaliadas por RM, é baixa para tempos de implantação entre 5 e 10 anos. Apesar desse achado positivo, a possibilidade de complicações silenciosas reforça a importância da vigilância clínica e da realização de estudos contínuos na área.
Limitações
A impossibilidade de precisar o momento exato da ruptura é uma limitação deste estudo, pois isso só seria possível por meio de exames periódicos de RM ao longo da vida útil do implante. Sendo assim, ao afirmar que houve suspeita de ruptura em 0,63% dos implantes inseridos há 9 anos, por exemplo, a hipótese de que as rupturas ocorreram antes dos 9 anos não pode ser descartada. Outra limitação do estudo é o tamanho amostral pequeno e o curto período de observação, que dificultam as conclusões. Com 161 pacientes e 322 implantes, a amostra pode não ser representativa da população geral de pacientes com implantes mamários. Além disso, a falta de dados sobre o acompanhamento em longo prazo pode limitar a compreensão completa da durabilidade dos implantes.
Referências
1. Goldwyn RM. Vincenz Czerny and the beginnings of breast reconstruction. Plast Reconstr Surg 1978;61(05):673-681. Doi: 10.1097/00006534-197805000-00003
2. Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Congress of Plastic Surgery. Amsterdam: Excerpta Medica Foundation; 1964:41-49
3. Pirjavec Mahić A, Grebić D, Čargonja P, Kustić D Silicone Gel Breast Implants: Past, Present, and Future. Acta Med Hist Adriat 2020;18 (01):165-176. Doi: 10.31952/amha.18.1.10
4. Yoon WJ. Endoscopic Transaxillary Augmentation Mammoplasty. New York: Springer; 2019
5. Dempsey WC, Latham WD. Subpectoral implants in augmentation mammaplasty. Plast Reconstr Surg 1968;42(06):515-521. Doi: 10.1097/00006534-196812000-00001
6. Griffiths CO. The submuscular implant in augmentation mammaplasty. In: Translations of the Fourth International Congress of Plastic Surgery. Amsterdam: Excerpta Medica Foundation; 1967: 1009-1015
7. Regnault P. Partially Submuscular Breast Augmentation. Plast Reconstr Surg 1977;59(01):72-76. Doi: 10.1097/00006534197701000-00013
8. Hoehler H. Breast augmentation: the axillary approach. Br J Plast Surg 1973;26(04):373-376. Doi: 10.1016/s0007-1226(73) 90044-1
9. Höhler H. Further progress in the axillary approach in augmentation mammaplasty: Prevention of incapsulation. Aesthetic Plast Surg 1976;1(01):107-113. Doi: 10.1007/BF01570242
10. Eiseman G. Augmentation mammaplasty by the trans-axillary approach. Plast Reconstr Surg 1974;54(02):229-232
11. Ho LCY. Endoscopic assisted transaxillary augmentation mammaplasty. Br J Plast Surg 1993;46(04):332-336. Doi: 10.1016/ 0007-1226(93)90015-4
12. Price CI, Eaves FF III, Nahai F, Jones G, Bostwick J III. Endoscopic transaxillary subpectoral breast augmentation. Plast Reconstr Surg 1994;94(05):612-619. Doi: 10.1097/00006534-199410000-00007
13. Di Pompeo FS, Paolini G, Firmani G, Sorotos M. History of breast implants: Back to the future. JPRAS Open 2022;32:166-177. Doi: 10.1016/j.jpra.2022.02.004
14. Mccarthy C. Too Young to Decide? The FDA’s Role in Regulating Breast Augmentation in Adolescents. Women Leading Change 2019;4(02):67-81
15. Perry D, Frame J. The history and development of breast implants. Annals of the Royal College of Surgeons of England 2020;102(07): 478-482. Doi: 10.1308/rcsann.2020.0003
16. International Society of Aesthetic Plastic Surgery. ISAPS International Survey on Aesthetic/Cosmetic Procedures Performed in 2023. ISAPS; 2024. Available from: https://www.isaps.org/media/rxnfqibn/isaps-global-survey_2023.pdf
17. Burciaga FJdlR, Rivas HAS, Preza LGG, Cervantes AFV, Gómez LDN. Breast Implant Rupture: Causes, Diagnosis and Treatment. Int J Med Sci Public Health 2023;3(05):857-860. Doi: 10.47191/ ijmscrs/v3-i5-13
18. Lake E, Ahmad S, Dobrashian R. The sonographic appearances of breast implant rupture. Clinical Radiology 2013;68(08):851-858. Doi: 10.1016/j.crad.2013.03.014
19. Salzman MJ. Silent Rupture of Silicone Gel Breast Implants: HighResolution Ultrasound Scans and Surveys of 584 Women. Plast Reconstr Surg 2022;149(01):7-14. Doi: 10.1097/PRS.000000000 0008632
20. Ayyala HS, Afifi T, McCarthy C, Cordeiro P. To screen or not to screen: Evaluation of Magnetic Resonance Imaging Surveillance to assess silicone breast implant integrity. Plast Reconstr Surg Glob Open 2022;10(4S):15-16. Doi: 10.1097/01.GOX.0000828108. 49655.84
21. Mazzocconi L, De Lorenzi F, Carbonaro R, Lorenzano V, Rotili A, Pesapane F, et al. Non-contrast MRI and post-mastectomy silicone breast implant rupture: preventing false positive diagnoses. Eur J Cancer Prev 2024;33(06):525-532. Doi: 10.1097/CEJ.00000000 00000887
22. Goldammer F, Pinsolle V, Dissaux C, Pélissier P. Accuracy of mammography, sonography and magnetic resonance imaging for detecting silicone breast implant ruptures: A retrospective observational study of 367 cases. Ann Chir Plast Esthet 2021;66 (01):25-41. Doi: 10.1016/j.anplas.2020.09.001
23. Food and Drug Administration (FDA) Breast Implants - Certain Labeling Recommendations to Improve Patient Communication. Guidance for Industry and Food and Drug Administration Staff. Rockville, MD: FDA; 2020. Available from: https://www.fda.gov/media/131885/download
24. Hölmich LR, Friis S, Fryzek JP, Vejborg IM, Conrad C, Sletting S, et al. Incidence of silicone breast implant rupture. Arch Surg 2003;138 (07):801-806. Doi: 10.1001/archsurg.138.7.801
25. Pitanguy I, Amorim NFGd, Ferreira AV, Berger R. Análise das trocas de implantes mamários nos últimos cinco anos na Clínica Ivo Pitanguy. Rev Bras Cir Plást 2010;25(04):668-674. Doi: 10.1590/ S1983-51752010000400019
26. Scaranelo AM, Marques AF, Smialowski EB, Lederman HM. Evaluation of the rupture of silicone breast implants by mammography, ultrasonography and magnetic resonance imaging in asymptomatic patients: correlation with surgical findings. Sao Paulo Med J 2004; 122(02):41-47. Doi: 10.1590/s1516-31802004000200002
27. Stevens WG, Calobrace MB, Alizadeh K, Zeidler KR, Harrington JL, D’Incelli RC. Ten-year core study data for sientra’s food and drug Administration-Approved round and shaped breast implants with cohesive silicone gel. Plast Reconstr Surg 2018;141(4S Sientra Shaped and Round Cohesive Gel Implants):7S-19S. Doi: 10.1097/PRS.0000000000004350
28. Spear SL, Murphy DKAllergan Silicone Breast Implant U.S. Core Clinical Study Group. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg 2014;133(06): 1354-1361. Doi: 10.1097/PRS.0000000000000021
29. Caplin DA, Calobrace MB, Wixtrom RN, Estes MM, Canady JW. MemoryGel Breast Implants: Final Safety and Efficacy Results after 10 Years of Follow-Up. Plast Reconstr Surg 2021;147(03): 556-566. Doi: 10.1097/PRS.0000000000007635
30. Fritsch H, Celik M, Warm M, Thangarajah F, Vogel-Minea C, Malter W, et al. Sonographic Assessment of Breast Implants Using Strain Elastography and Shear Wave Elastography in an Animal Model. Anticancer Res 2024;44(02):497-501. Doi: 10.21873/anticanres.16837
31. Di Benedetto G, Cecchini S, Grassetti L, Baldassarre S, Valeri G, Leva L, et al. Comparative Study of Breast Implant Rupture Using Mammography, Sonography, and Magnetic Resonance Imaging: Correlation with Surgical Findings. Breast J 2008;14(06): 532-537. Doi: 10.1111/j.1524-4741.2008.00643.x
32. Maijers MC, Niessen FB, Veldhuizen JFH, Ritt MJPF, Manoliu RA. MRI screening for siliconebreast implant rupture: accuracy, interand intraobserver variability using explantation results as reference standard. Eur Radiol 2014;24(06):1167-1175. Doi: 10.1007/s00330-014-3119-8
33. Lotan AM, Retchkiman M, Tuchman I, Binenboym R, Gronovich Y. Analysis of 109 Consecutive Explanted Breast Implants: Correlation Between Suspected Implant Rupture and Surgical Findings. Aesthetic Plast Surg 2016;40(05):739-744. Doi: 10.1007/s00266016-0689-7
34. Haws MJ, Alizadeh K, Kaufman DL. Sientra primary and revision augmentation rupture trending and analysis with magnetic resonance imaging. Aesthet Surg J 2015;35(Suppl 1):S33-S42. Doi: 10.1093/asj/sjv021
35. Paolini G, Firmani G, Briganti F, Macino M, Nigrelli S, Sorotos M, Di Pompeo FS. Assessment of Risk Factors for Rupture in Breast Reconstruction Patients with Macrotextured Breast Implants. Aesthetic Plast Surg 2023;47(02):517-530. Doi: 10.1007/ s00266-022-03118-9
36. Larsen A, Bak EEF, Hart LB, Timmermann AM, Ørholt M, Weltz TK, et al. Silicone Leakage from Breast Implants Is Determined by Silicone Cohesiveness: A Histologic Study of 493 Patients. Plast Reconstr Surg 2024;154(06):1159-1171. Doi: 10.1097/PRS.00 00000000011395
37. Kinney BM, Jeffers LLC, Ratliff GE, Carlisle DA. Silicone gel breast implants: science and testing. Plast Reconstr Surg 2014;134(1, Suppl)47S-56S. Doi: 10.1097/PRS.0000000000000349
38. Lindenblatt N, El-Rabadi K, Helbich TH, Czembirek H, Deutinger M, Benditte-Klepetko H. Correlation between MRI results and intraoperative findings in patients with silicone breast implants. Int J Womens Health 2014;6:703-709. Doi: 10.2147/IJWH. S58493
39. Kim HB, Han HH, Eom JS. Magnetic Resonance Imaging Surveillance Study of Silicone Implant-based Breast Reconstruction: A Retrospective Observational Study. Plast Reconstr Surg Glob Open 2023;11(06):e5031. Doi: 10.1097/GOX.0000000000005031
40. Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017;6(02):163-168. Doi: 10.21037/gs.2016.09.12
41. Fleury EdFC. Breast silicone implants’ pericapsular impairment: current underdiagnosed status. Front Surg 2023;10:1249078. Doi: 10.3389/fsurg.2023.1249078
1. Especialidade de Cirurgia Plástica, Clínica Particular, Rio de Janeiro, RJ, Brasil
Endereço para correspondência Wanda Elizabeth Massiere-y-Corrêa, Especialidade de Cirurgia Plástica, Clínica Particular, Rio de Janeiro, RJ, Brasil (e-mail: wandaelizabethcorrea@gmail.com pesquisa.clinica@silimed.com.br).
Artigo submetido: 10/11/2023.
Artigo aceito: 24/03/2025.
Conflito de Interesses
A autora declara que este estudo foi totalmente patrocinado pela Silimed.




 Read in Portuguese
Read in Portuguese
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter