

Review Article - Year 2025 - Volume 40Issue 1
Burn Wound Infections: A 35-year Review of Advances, Diagnostic Challenges, and Evidence-based Strategies
Infecções em feridas em queimados: Revisão de 35 anos de avanços, desafios diagnósticos e estratégias baseadas em evidências
ABSTRACT
Introduction Severe burns represent a critical public health issue, with high mortality and morbidity associated with wound infections, which are the leading cause of death in these patients. The present article aims to provide a comprehensive evidence-based approach for the diagnosis and management of invasive burn wound infections, highlighting recent scientific advances and effective clinical strategies.
Materials and Methods A thorough review of 35 years of scientific literature was conducted using recognized databases such as PubMed, EMBASE, and Cochrane Library. The information was synthesized and validated by a committee of experts in surgery and burn care. The medical subject heading (MESH) terms used were: Burns, Infection, Burn wound infections, Sepsis, and Multi-drug resistant.
Results The analysis shows that more than 45% of patients with severe burns develop infections. The pathophysiology includes immunosuppression secondary to hypermetabolism and inflammatory dysregulation, which predispose patients to infections by multidrug-resistant microorganisms. Additionally, biomarkers such as procalcitonin and strategies based on specific cultures improve diagnostic accuracy. The implementation of personalized protocols reduces complications and mortality in this population.
Conclusion The present study establishes an evidence-based guide for the early diagnosis and effective management of burn infections, promoting the rational use of antibiotics and comprehensive care. The findings contribute to standardizing clinical practices, optimizing hospital resources, and improving clinical outcomes. This review serves as a crucial reference for advancing burn patient care and fostering future research in the field.
Keywords: burns; burn units; surgery department; sepsis; hospital infections; drug resistance
RESUMO
Introdução As queimaduras graves representam um problema crítico de saúde pública, com altas taxas de mortalidade e morbidade associadas às infecções em feridas, a principal causa de morte nesses pacientes. Este artigo tem como objetivo fazer uma abordagem abrangente baseada em evidências ao diagnóstico e manejo de infecções invasivas em feridas por queimaduras, destacando avanços científicos recentes e estratégias clínicas eficazes.
Materiais e Métodos Foi realizada uma revisão abrangente de 35 anos de literatura científica utilizando bases de dados reconhecidas, como PubMed, EMBASE e Cochrane Library. As informações foram sintetizadas e validadas por um comitê de especialistas em cirurgia e cuidados com queimados. Os termos MESH utilizados foram Queimaduras, Infecção, Infecções em feridas por queimaduras, Sepse, e Multirresistência a medicamentos.
Resultados A análise mostra que mais de 45% dos pacientes com queimaduras graves desenvolvem infecções. A fisiopatologia inclui imunossupressão secundária ao hipermetabolismo e à desregulação inflamatória, que predispõe os pacientes a infecções por microrganismos multirresistentes. Biomarcadores como a procalcitonina e estratégias baseadas em culturas específicas melhoram a precisão diagnóstica. A implementação de protocolos personalizados reduz as complicações e a mortalidade nessa população.
Conclusão Este estudo estabelece um guia baseado em evidências para o diagnóstico precoce e manejo eficaz das infecções em queimaduras, promovendo o uso racional de antibióticos e cuidados abrangentes. Os achados contribuem para a padronização das práticas clínicas, otimização dos recursos hospitalares e melhora dos desfechos clínicos. Esta revisão é uma referência crucial para o avanço no cuidado de pacientes queimados e estímulo de futuras pesquisas na área.
Palavras-chave: queimaduras; unidades de queimados; cirurgia plástica; sepse; infecções bacterianas; resistência microbiana a medicamentos
Introduction
The World Health Organization (WHO) estimates that ~ 180 thousand people die each year due to burns, a problem that predominantly affects lowand middle-income countries. In addition to fatalities, non-lethal burns are a significant cause of morbidity, leaving thousands with physical and psychological sequelae that affect their quality of life. These incidents often occur in everyday spaces, such as homes and workplaces, where risks are elevated, and safety measures are often insufficient. Despite their severity, most burns are preventable with adequate prevention strategies and safety education in these environments.
In lowand middle-income countries, burns rank among the leading causes of disability-adjusted life years (DALYs) lost. Hospitalization for burns varies by country and health coverage programs. However, studies indicate a global trend toward shorter hospital stays and increased treatment of burns in specialized centers across many countries.1
In patients with severe burns, infections represent one of the main threats to life, particularly in those who have survived the initial resuscitation phase. In this context, infections not only become the leading cause of mortality but also present complex challenges in terms of diagnosis and effective treatment. Therefore, this document becomes essential to explore various critical aspects related to invasive infections in burn wounds.
Burn patients undergo specific physiological changes due to their thermal trauma response. The inflammatory response triggered by burns inherently leads to immunosuppression, increasing the risk of infections, particularly affecting the tissue involved in the wound. Additionally, the variety of infectious complications and their potential manifestations pose challenges for timely diagnosis and treatment.2 Finally, specific strategies for clinical management are proposed with the aim of improving prognostic outcomes for this special group of patients.
The indiscriminate use of broad-spectrum antibiotics in burn patients can have several adverse effects, such as promoting bacterial translocation, increasing the risk of infections in distant organs like the liver and lungs.3 Furthermore, the use of antibiotics can disrupt the intestinal flora, leading to increased intestinal permeability and further promoting translocation, compromising the host’s defenses.4 Another significant effect is the increased risk of colonization or infection by multidrug-resistant organisms related to prior exposure to antibiotics such as extended-spectrum cephalosporins and carbapenems. Associations have been found between these antibiotics and an increased risk of infection by multidrug-resistant gram-negative bacteria in critically ill burn patients,5 as well as the induction of resistance in enterobacteriaceae like Escherichia.6 On the other hand, the use of antibiotics with high sodium content can contribute to hypernatremia in patients with severe burns, further complicating the clinical management of these patients.7
Thus, burn wound infection is the most severe complication, as it significantly prolongs hospitalization and worsens both aesthetic and functional outcomes. It also greatly increases sequelae and costs due to the need for additional surgeries, anesthesia, antibiotics, and hospital time, notably increasing the patient’s risk of mortality.8
While in other infection sites extended studies are required to locate the infection, in burn patients, the primary infection site is easily accessible-the injured skin-so daily physical examinations allow for early detection and anticipation of infection progression. Timely diagnosis and treatment are crucial to prevent the spread of infection, and visible clinical characteristics are key to identifying the problem before laboratory results are available. Therefore, a serial physical examination performed by a trained professional is essential for success.8
Materials and Methods
The present study consisted of a systematic review of the available literature over the past 35 years, focused on invasive infections in burn wounds. The methodology was developed following the steps outlined below:
Sources of information: Internationally recognized databases such as PubMed, EMBASE, and Cochrane Library were used, along with guidelines and recommendations from the International Society for Burn Injuries, the American Burn Association, and other specialized organizations.
Inclusion and exclusion criteria: Studies published between 1988 and 2024 that addressed epidemiology, pathophysiology, diagnosis, and management of infections in burn patients were included. Peer-reviewed articles, clinical guidelines, meta-analyses, and prospective cohorts were prioritized. Studies with insufficient samples, those without full text access, or those that did not meet methodological quality standards were excluded.
Selection process: Titles and abstracts identified in the initial search were evaluated by two independent reviewers. Those that met the criteria were analyzed in depth. Discrepancies were resolved by consensus with a third reviewer.
Data synthesis: The extracted data were categorized into four key areas:
Epidemiology and risk factors;
Pathophysiology;
Diagnostic tools (biomarkers, cultures);
Therapeutic strategies (rational use of antibiotics).
Validation and review: The content was presented and discussed with a multidisciplinary group of experts in surgery and burn care, who made final adjustments to ensure the clinical applicability of the findings. This methodology allowed for the integration of current knowledge with clinical practices, providing a solid framework for the creation of an evidence-based guide.
Epidemiology
The incidence of infections in burn patients’ wounds varies depending on the study and the population analyzed. A retrospective study conducted in a burn intensive care unit found that 45.8% of patients developed infections, with an infection rate of 45.8 per one thousand patient-days.9 The progression of infections toward sepsis is a significant phenomenon. According to the study by Belba et al., the prevalence of sepsis in adult burn patients was 26%, with an accumulated incidence of 30 patients per one hundred adults.10 The highest rates of burn wound infections occur in the lower extremities, but specific pathogens are not limited to any particular anatomical location.11
Burn patients also have high rates of other types of infections, such as catheter-related infections, bacteremia, and pneumonia, but wound infections rank first.11,12 In a study with a cohort of 175 patients with severe burns, wound infections preceded multiorgan dysfunction in 83% of patients and were considered the direct cause of death in 36% of those who died.13 Studies have linked infection in general as the leading cause of mortality in burn patients, being responsible, either directly or indirectly, for 33 to 80% of deaths.14,15
Pathophysiology
When any type of wound occurs, a series of cellular and biochemical events are triggered, with the final goal being wound closure. Healing can be divided into three overlapping phases: inflammation, proliferation, and remodeling.16
Depending on the degree of the burns, the tissue restitution time will vary, directly affecting the aesthetic outcome and increasing the exposure of the tissue usually protected by the epidermis, making it more susceptible to infection.
Superficial (grade I): Only the epidermis is affected. For example, sunburns, healing in 7 to 10 days.
Partial-thickness superficial and deep (grade II): Affects various degrees of the dermis. When superficial, it is called type A, healing without significant sequelae in less than 14 days. When the intermediate dermis is involved, it is considered type AB, healing after 18 days, resulting in poor-quality scars, with the appearance of keloids, hyper or hypopigmentation, and retractions.
Full-thickness (grade III): Destroys the entire dermis, preventing epithelialization, also known as type B, healing by secondary intention, requiring debridement until granulation tissue is obtained and grafting.
Grade IV: Involves the destruction of muscle and/or bone structures, generally resulting from electrical burns.
Infection in a burn can even compromise the unaffected dermis from the initial injury, preventing epithelialization and worsening the lesion. For example, a superficial seconddegree burn can rapidly deepen if not properly managed, transforming into a full-thickness burn in a few days, prolonging recovery time and requiring debridement and grafting instead of healing in less than 15 days.8
An important effect of burn injuries is the deregulated inflammatory response that can progress to a state of immunosuppression. In experimental mouse models with burns, when leukocyte populations from severely burned mice were transferred to healthy mice, they altered pathogen recognition and negatively affected the balance between regulatory T cells and helper T cells.17
Biochemically, in response to tissue injury, molecules are activated that trigger the immune system, especially damage-associated molecular patterns (DAMPs) and pathogenassociated molecular patterns (PAMPs). An example of the latter includes lipopolysaccharides from the cell wall of gram-negative bacteria, viral double-stranded RNA, and flagellin. Initially, DAMPs emerge as intracellular proteins, uric acid, extracellular DNA, and ATP released by affected cells in the burned tissue, entering the systemic circulation and activating recognition signals mediated by Toll-like receptors (TLRs). These are molecules expressed on circulating leukocytes and various skin cells, including keratinocytes, Langerhans cells, T and B cells, mast cells, endothelial cells, myofibroblasts, and primary fibroblasts, with the primary function being the immunological stimulation for cytokine secretion to protect the damaged tissue from potential infections and participate in the repair of the damaged skin. In severe burns, this process reaches extreme levels, affecting the immune system, even interfering with healing and increasing the risk of infection. This disruption in homeostasis is directly proportional to the severity and extent of the burn, which can trigger a cytokine storm with immune system paralysis, resulting in decreased production of interleukins (IL)-6 and IL-12, leading to reduced antigen presentation and decreased T cell proliferation, two key processes in a regulated immune response. This inflammatory process can progress to multiorgandysfunction syndrome and death. 1,17,18
In a large-scale burn study, it was found that when the total body surface area (TBSA) burned exceeded 20%, the gene expression of white blood cells changed drastically (80% of genes), a phenomenon known as a “genomic storm”.19 The DAMPs that activate TLRs and other pattern recognition receptors can be classified into:
1. Proteins expelled through secretory lysosomes, such as high mobility group box (HMGB1) and galectin-3.
2. Molecules released by necrotic cells such as S100 proteins, HMGB1, IL-1a, galectin-3, HSP60, HSP70, HSP72, histones, and nucleic acids.
3. Molecules from the extracellular matrix like hyaluronic acid, heparan sulfate, fibronectin, and degraded matrix components.
Although numerous DAMPs have been identified, theoretically, any molecule that normally resides inside cells and is expelled or altered by tissue damage can act as a DAMP. It has been proposed that hydrophobic surfaces in general act as DAMPs.
On the other hand, PAMPs come from pathogens and the skin microbiota that enter the dermis through the broken epidermal barrier. In normal skin, bacterial load has been quantified using quantitative real-time polymerase chain reaction (16S rRNA gene) in deep epidermal layers (punch biopsies), intermediate layers (scrapings), and epidermal surfaces (swabs), with 10 thousand; 50 thousand; and 1 million colony-forming units (CFU) per square centimeter, respectively,20 of bacteria from various species.21 Therefore, once the epidermis is affected by the burn, these bacterial PAMPs, like DAMPs, activate TLR signaling, which converges on a common pathway, with more power in the area surrounding hair follicles, as it is the microenvironment where bacteria concentrate.22 As the exposure continues due to the defect in the epidermal barrier, pathogens that colonize the surface have the opportunity to infiltrate the wound and develop an infection.
Exposure to pathogens occurs not only through the burn wound but also through invasive devices, and bacterial translocation from the gastrointestinal tract has even been described.23Thesemicroorganismscancome from the patient’s endogenous microbiota (skin, intestine, and upper respiratory tract) as well as from contaminated external sources, such as the hospital environment and healthcare workers,the latterassociated with cross-contamination, which extends the appearance and spread of antimicrobial resistance, posing a severe threat to healthcare,24,25 particularly among critically ill patients.
On the other hand, the indiscriminate use of antibiotics, combined with water loss from the evaporation of the injured epidermis, increases sodium concentration. A 10% increase in sodium concentration stimulates keratinocytes to secrete higher amounts of TLRs, enhancing the described inflammatory response. The burn eschar also contributes to inflammatory signaling and healing, at least partially acting as a reservoir for DAMPs and PAMPs. It has been found that patients with burns involving more than 50% of the TBSA who are fully debrided and covered within 3 days of the injury have an approximate 40% reduction in basal metabolic rate, preventing further net protein loss from muscle catabolism and a decrease in bacterial load in cultures, having a 30% lower risk of developing sepsis, thus reducing mortality.26
Another phenomenon associated with eschar formation is biofilm development. These are microbial communities that adhere to the surfaces of wounds and are protected by an extracellular matrix, making them resistant to antibiotics and the host’s immune system.27 It has been identified that serum from patients with severe burns can increase biofilm formation due to oxidative stress, which worsens infections and complicates wound management.28
The glycocalyx, a gel-like layer covering the luminal surface of vascular endothelial cells, composed of proteoglycans bound to the membrane, glycosaminoglycan chains, glycoproteins, and attached plasma proteins, plays a crucial role in vascular homeostasis, regulating permeability, microvascular tone, preventing thrombosis, and modulating leukocyte adhesion.29 When degradation of this protective structure occurs, known as “shedding,” exacerbated by the inflammatory response and oxidative stress associated with thermal trauma in patients with severe burns, it increases vascular permeability, directly facilitating microbial invasion (biofilm formation) and indirectly allowing the access of immune cells and harmful agents (such as proteases and reactive oxygen species), which enhance endothelial damage and predispose to infections. Endothelial dysfunction manifests through the release of components such as syndecan-1 and heparan sulfate into the bloodstream, which has been correlated with burn severity,27-30 being more pronounced in older patients and those with more extensive burns, suggesting a relationship between injury severity and the degree of glycocalyx shedding.31
Risk Factors
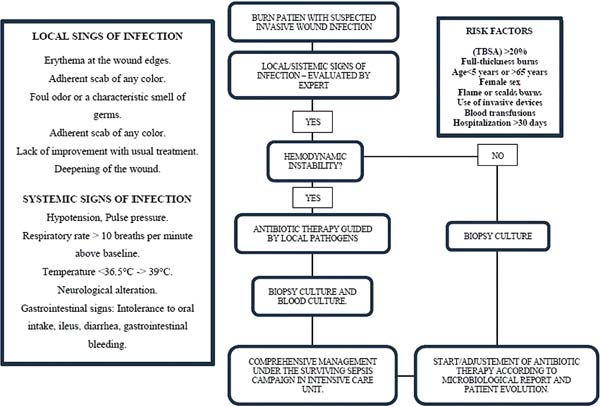
Burn patients are particularly predisposed to infections due to the loss of skin integrity, immunosuppression, and the need for invasive procedures. Various studies have described risk factors for infections in these patients, which can be classified into three main groups.
1. Patient-related factors:
Advanced age: Both early (< 5 years) and advanced age have been associated with an increased risk of infections in burn patients, with the risk increasing from age 50, peaking at age 65.32
Female sex: The female sex has been identified as an independent predictor of infections in burn patients, usually associated with the nature of burns due to domestic tasks.33
2. Extent and depth of the burn: Total bodysurfaceareaburned: ATBSAburngreater than 20% is significantly associated with a higher risk of infections.34 Full-thickness burns: The presence of full-thickness burns is an independent risk factor for wound infections.32 Inhalation injury: Inhalation injury increases the risk of infections associated with medical care, such as healthcare-associated pneumonia.34
3. Hospital interventions:
Use of catheters: The insertion of urinary, arterial, and central venous catheters is associated with an increased risk of colonization and infection by multidrug-resistant gram-negative organisms. The use of central venous catheters has also been identified as an independent risk factor for infections.35,36
Mechanical ventilation: Mechanical ventilation is a significant risk factor for respiratory infections in critically ill burn patients.36
Blood transfusions: The administration of blood products is associated with a higher risk of infections.33-36 Hospitalization duration: Prolonged hospitalization (>30 days) correlates with a higher risk of infection, including bacteremia and fungemia.37,38
4. Other factors: Sepsis: The presence of sepsis is an independent predictor of recurrent infections in burn patients.33
Surgical interventions: Procedures such as burn excision and skin grafting increase the risk of recurrent infections.33 Hydrotherapy: Hydrotherapy has been associated with a higher risk of infections.38
The systemic severity of a burn is, therefore, multifactorial. For example, a 40% TBSA burn carries an 11% mortality risk in a 20-year-old patient, whereas the same burnin a 65-year-old patient has a mortality risk over 80%.37 Identifying and managing these risk factors is crucial for improving clinical outcomes and reducing the incidence of infections in this vulnerable population.
Diagnostic Approach
There are well-defined criteria for diagnosing various infections in non-burn patients. However, in burn trauma, as mentioned earlier, a hypermetabolic response is triggered that differs from any other type of injury. For example, the normal basal temperature in a patient with severe burns rises to 38.5°C instead of 37.5°C, as in typical patients. Metabolismhasbeenidentifiedtoincreasebyup to 180%,26 and heart and respiratory rates may remain elevated for weeks or even months. Considering these factors, the American Burn Association held a consensus meeting in 2007 regarding sepsis in burn patients, defining a guide to establish high suspicion criteria described below.39
Subsequent studies and classifications have been developed to seek criteria with better diagnostic performance for sepsis in burn patients. In 2018, Yan et al.38 published a prospective cohort study aimed at validating current sepsis scores for burn patients. This study evaluated the accuracy of 3 sepsis diagnostic criteria scales for burn patients and compared the results: the 2007 American Burn Association (ABA) criteria, Mann-Salinas 2013 sepsis predictors, and the 2016 Sepsis-3 definition from the Surviving Sepsis Campaign. The study included 418 patients with severe burns treated at a hospital in Toronto between 2006 and 2016, 21% of whom developed sepsis. Sepsis diagnoses were made prospectively, considering specific clinical signs and using selected criteria to compare their predictive capabilities.
The results indicated that the Sepsis-3 criteria had the highest accuracy, identifying 85% of sepsis cases, compared with 59% for the ABA criteria and 28% for Mann-Salinas. However, none of the criteria evaluated showed sufficient reliability to be a diagnostic standard for this specific population. The authors recommend that the diagnosis of sepsis in burn patients be based on a comprehensive clinical assessment performed by a specialized burn care team, rather than relying solely on predictive criteria.38
It is essential that the treating surgeon be trained to clinically identify an infected burn, as early and accurate diagnosis is crucial to avoid serious complications. Understanding key concepts allows differentiation between the normal course of wound healing and signs indicative of infection, providing clinicians with the necessary tools to effectively discern the presence of infection. This ability not only improves treatment outcomes but also contributes to better resource management and avoids unnecessary interventions. Therefore, it is crucial to define fundamental concepts that will alter the patient’s therapeutic course.
- Colonization: Refers to the presence of fewer than 100 thousand germs per gram of tissue without evidence of invasion or systemic signs of infection. This colonization could have beneficial effects, as it may stimulate growth factors, cytokines, and the cellular immune response.
- Non-invasive infection: Occurs when there are more than 100 thousand germs per gram, but without invasion into the underlying healthy tissue. This bacterial level may cause deterioration of the wound or failure in graft integration. The patient is considered infected and should be evaluated for the risk of invasive infection and sepsis.
- Invasive burn wound infection: Refers to a wound with local or systemic signs of infection and more than 100 thousand germs per gram of tissue, compromising the underlying healthy tissue.
- Cellulitis: An inflammatory process in the wound area, characterized by erythema and edema that exceed the normal boundaries, usually associated with other local and systemic signs.
- Ecthyma gangrenosum: Presents as necrotic ulcers with well-defined borders and an inflammatory halo that arises at the base of the wound.
-Impetigo: A late lesion observed in previously epithelialized areas, such as between already integrated grafts or in donor sites that have already epithelialized.
Local signs of infection:
Erythema at the wound edges.
Adherent scab of any color.
Foul odor or a characteristic smell of germs.
Lack of improvement with standard treatment.
Deepening of the wound.
Systemic signs of infection: The systemic signs are similar to those of other infections, but in burn patients, there are special considerations for diagnosis and management:
Vital signs:
- Blood pressure: Unexplained drops in mean or diastolic blood pressure are signs of vasodilation associated with infection.
- Respiratory rate: An increase of more than 10 breaths per minute above baseline may indicate infection.
- Temperature: Increase or decrease in body temperature (36.5°-38.5° C) and fever above 39° C are indicative of infection. Hypothermia suggests a gram-negative pathogen.
- Neurological: Altered consciousness without a justified cause may indicate infection.
- Gastrointestinal signs: Nutrition intolerance, ileus without abdominal injury, unexplained diarrhea, or gastrointestinal bleeding are signs of uncontrolled infection.
In burn patients, leukocytosis is often present with slightly elevated white blood cell levels, between 10 thousand and 11 thousand cells per microliter. Values higher than 12 thousand require investigation of a potential infection focus, such as unresected necrosis or infection by gram-positive cocci, especially staphylococci.
On the other hand, leukopenia is a concern in these patients, especially when there is a sudden drop in the leukocyte count from baseline. For example, a blood test showing 6 thousand leukocytes and 60% neutrophils may be normal in a healthy individual, but in a burn patient, it indicates a low count. If 48 hours earlier the count was high (15 thousand leukocytes and 85% neutrophils), followed by leukopenia and neutropenia, it may indicate sepsis associated with gram-negative organisms. However, it should be noted that silver sulfadiazine can induce neutropenia and act as a distractor.
Finally, thrombocytopenia with a platelet count below 100 thousand per microliter may indicate infection by consumption, especially in infections related to Staphylococcus aureus, potentially progressing to disseminated intravascular coagulation.8-40
SkinCulture and Biopsy
Surgical technique: Involves excising a 1 cm x 0.5 cm piece of skin without subcutaneous tissue and determining the number of germs per gram of tissue. A biopsy culture with more than 100 thousand CFU per gram of tissue is considered microbiological criteria for invasive infection.8
Regarding wound colonization, a study conducted in the burn unit of the Hospital Universitario del Valle, Cali- Colombia collected paired samples and showed that surface culture coincided with biopsy culture in only ~ 20% of cases. This means that administering antimicrobials based on a surface culture involves a risk of error of nearly 80%.41
Furthermore, positive blood cultures in burn patients should not be considered contaminants due to the immunosuppression described. A positive blood culture associated with clinical signs of infection is an indication to begin specific antimicrobial treatment as soon as possible. The Infectious Diseases Society of America (IDSA) and the American Society for Microbiology advise against collecting samples with swabs due to significant limitations such as: (1) high risk of surface and subcutaneous contamination, and (2) limited sample capacity (500 μL), leading to insufficient sample quantity, especially when cultures other than bacteriological (fungal, mycobacterial) are requested. Additionally, before any sampling or biopsy, the wound must be thoroughly cleaned and free of topical antimicrobials and residues that could affect culture results.42
The approximate chronological aspects of wound colonization and infection processes in burn wounds and the characteristics of the microorganisms involved have been identified. Initially, with normal flora predominantly consisting of gram-positive cocci, bacterial invasion by gram-negative bacteria, some of which come from the hospital environment, starts between 2 to 4 days. Finally, fungi such as Candida sp. and multidrug-resistant bacteria can invade the wound.
Clinical Characteristics of Each Pathogen
Some pathogens exhibit important clinical characteristics that should be detected during the physical examination, as they help raise clinical suspicion before laboratory results are obtained.
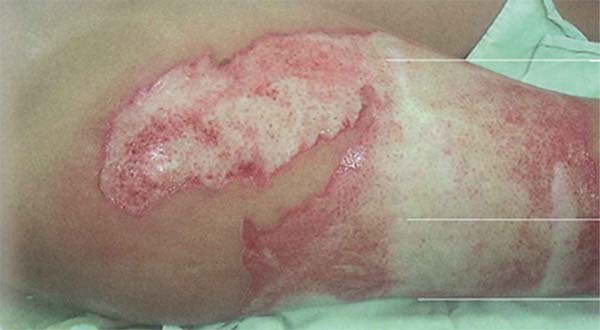
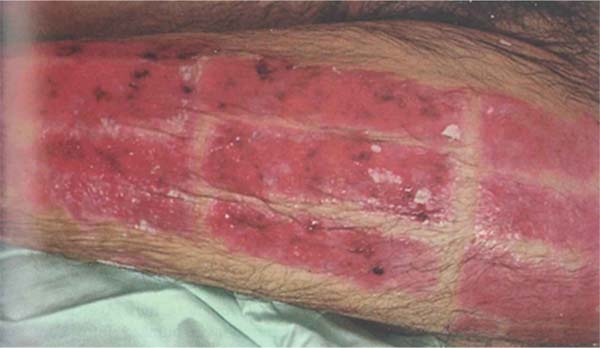
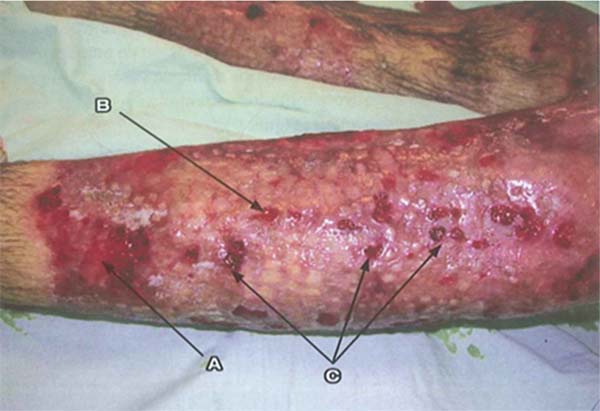
Pseudomonas aeruginosa: Enterobacteria, particularly P. aeruginosa, is characterized by the onset of leukopenia, as mentioned earlier, which can progress to multiple organ dysfunction. Therefore, if the leukogram is suspicious, showing leukopenia, signs specific to this pathogen should be sought in the wound, such as blue/green or intense yellow/green tissue (pyocyanin and pyoverdine), petechial hemorrhagic speckling, positive fluorescence, and a characteristic sweet odor, described as corn or grapes.8 The development of severe septic shock, accompanied by a wound turning gray, purple, or black, is indicative of invasive P. aeruginosainfection, known as “ecthyma gangrenosum.” These patients require immediate excision of the affected tissue, as the condition is associated with high mortality.43 (►Fig. 1; ►Fig. 2.)
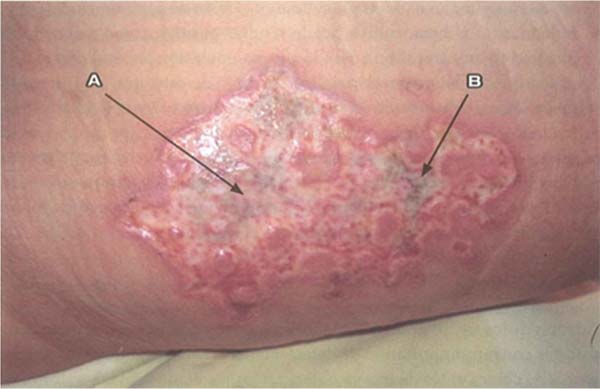
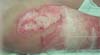
S. aureus: Unlike P. aeruginosa, S. aureus has a slower progression and is characterized by leukocytosis instead of leukopenia. Progression to multiple organ failure is less likely in the early stages. Wounds infected by this pathogen are characterized by findings like paleness of granulation tissue, depressed granulation tissue, and the appearance of pustules or comedones. It is characterized by multiple small superficial abscesses that tend to merge. This type of infection can lead to the breakdown of previously healed epithelialized areas and compromise already integrated grafts. Local treatment involves wound cleansing, debridement, and mupirocin application, alongside systemic antibiotic therapy. (►Fig. 3; ►Fig. 4.)
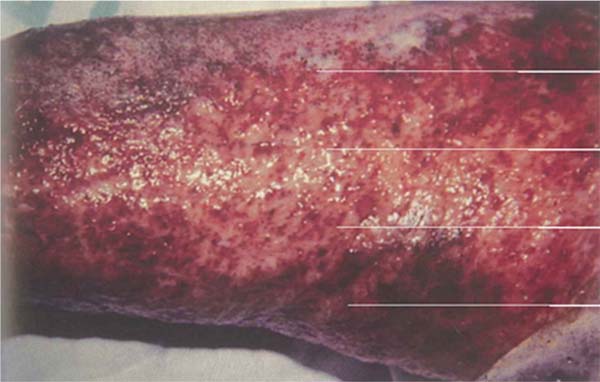
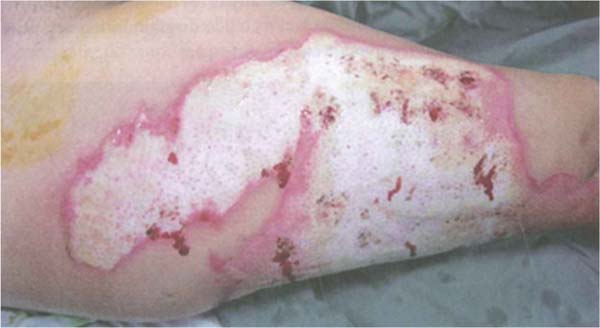
Streptococcus pyogenes: This infection presents with cellulitic lesions characterized by erythema, edema, and hyperesthesia in the area adjacent to the burn, donor site, or grafted area. When it affects a grafted area, the grafts may appear destroyed or absent the next day. Cellulitis can be caused by a variety of pathogens, but in burn wounds, the most common pathogen is group A β-hemolytic streptococcus. It should not be confused with early cellulitis related to trauma, where there is a perilesional erythematous reaction of 5 mm to 1 cm. When it is more extensive and associated with pain and fever, bacterial cellulitis should be suspected. Management includes washing, debridement, and immediate tangential excision of the wound in suspected areas. The area of cellulitis without an open wound should not be excised. (►Fig. 5; ►Fig. 6.)
Laboratory Studies
Li et al.44 conducted a meta-analysis that reviewed all available published data on biomarkers for the early detection of sepsis in hospitalized burn patients. The study encompassed 28 research papers assessing 57 distinct biomarkers and included a total of 1,517 participants. Given the inflammatory and hypermetabolic response triggered by thermal injuries, the article explores the diagnostic accuracy of these biomarkers, highlighting the challenges associated with early sepsis detection in burn patients, Of the 57 biomarkers evaluated, procalcitonin (PCT) showed moderate sensitivity (73%) and specificity (75%), while C-reactive protein (CRP) had high sensitivity (86%) but low specificity (54%). Other biomarkers such as brain natriuretic peptide, stroke volume index, TNF-α, and free DNA showed potential in isolated studies, but further research is needed to confirm their utility. The need to standardize evaluation approaches, considering sampling times, cut-off points, and sepsis definitions, is emphasized. The study concludes that no single biomarker is reliable enough to diagnose sepsis, recommending the exploration of combinations of biomarkers and standardized methodologies.
While relying on a single biomarker for diagnosis is not effective, most studies endorse their use in monitoring the progression of sepsis, as their levels are initially elevated and gradually decline as the condition resolves. Nevertheless, further prospective research is required to determine the most precise biomarkers for tracking sepsis progression.44 It is important to recognize that procalcitonin (PCT) has limited utility in the early diagnosis and management of sepsis, As it is secreted within three hours of endotoxin exposure but reaches its peak ~ 14 hours later.44,45 However, a 2015 meta-analysis evaluated the effectiveness of PCT as a biomarker to differentiate septic from non-septic patients in burn cases. It analyzed previous studies measuring PCT levels and their diagnostic capacity, determining an area under the curve (AUC) of 0.83 and a significant effect of sepsis on PCT levels (Cohen’s d ¼ 2.1; 95%CI: 1.1-3.2). With an estimated cut-off value of 1.47 ng/mL, the results highlight the usefulness of PCT to guide antimicrobial management, promoting informed decisions about the initiation and discontinuation of therapies, thereby improving clinical outcomes in burn patients.46
In 2022, a new meta-analysis was published that evaluated the utility of PCT to diagnose sepsis in adults with burns, analyzing 15 studies selected from 856 found. The results showed a sensitivity of 0.78, specificity of 0.85, and an area under the curve (AUC) of 0.88, indicating good early diagnostic performance. Although PCT shows potential as a marker for sepsis in this context, more high-quality studies are needed to confirm its clinical value.47
Overall, current evidence does not support the use of a single biomarker for sepsis diagnosis, as significant variability exists among published studies regarding patient populations, sepsis definitions, diagnostic thresholds, and reported outcomes. However, when accessible, these biomarkers can play a valuable role in guiding the initiation, monitoring, and discontinuation of antimicrobial therapy.
International Guidelines
International Society for Burn Injuries (ISBI) Recommendations Formulation 2022
On August 28, 2022, during the biennial ISBI meeting in Guadalajara, Mexico, based on the Surviving Sepsis Campaign strategy, a consensus was developed on burn-related sepsis statements, resulting in a series of recommendations.48
Use of Systemic Inflammatory Response Syndrome (SIRS) as a Method of Detecting Sepsis in Burn Patients
Systemic inflammatory response syndrome features are evident in all major burns, as leukocytes are continuously recruited to the wound, leading to fluctuations in white blood cell counts, either increased or decreased. These physiological alterations often result in positive SIRS scores, even in the absence of infection. Patients with burns covering more than 20% of the TBSA experience a pronounced hypermetabolic response, characterized by an elevated baseline temperature, tachycardia, and tachypnea. Therefore, they recommend not using SIRS as a method for detecting sepsis, but instead adhering to the definition of sepsis. Recommendation 1a strong, high-quality evidence.48
In this context, the Sequential Organ Failure Assessment (SOFA) score should serve as a foundation for developing a more refined set of criteria tailored to burn patients, As the current version does not adequately account for all organ systems commonly involved in burn-related sepsis, including the gastrointestinal, dermal/wound, and endocrine systems. Refining existing parameters, like the Glasgow Coma Scale, could enhance its utility. For instance, fluctuations in blood glucose levels exceeding 150 mg/dL, wound alterations (in appearance or odor), graft failure, and ileus are commonly linked to burn sepsis. During the resuscitation phase, elevated serum lactate levels and their clearance serve as prognostic markers but are not definitive indicators of sepsis.48
Routine use of any criteria to diagnose sepsis within the first 72 hours is not recommended. However, this does not mean that sepsis does not occur in burn patients, so the attending professional must identify alarm signals during that period; the best criterion is that of the clinical professional.
Several clinical and paraclinical factors have been identified as key indicators for suspecting sepsis in burn patients, including an increase of 2: 2 points in the SOFA score, lactate elevation > 2 mmol/L (> 18 mg/dL) as a proxy for base deficit, temperature fluctuations such as new-onset fever or hypothermia (with no established threshold), a sudden decline in platelet count, reduced urine output or rising fluid requirements, and acute kidney injury of stage 2: 1 based on the Kidney Disease: Improving Global Outcomes (KDIGO) criteria. Other relevant factors include respiratory disturbances, altered mental status, gastrointestinal dysfunction, wound changes suggestive of infection, and a procalcitonin increase of 2: 2 ng/mL from baseline. No single test provides a definitive sepsis diagnosis in burn patients. Instead, a combination of these factors proves more effective in prompting diagnostic or empirical treatment decisions. Consequently, laboratory results should be interpreted in the context of overall trends rather than isolated values, with any changes assessed relative to the patient’s baseline progression.48
There is currently no consensus on the exact triggering factors for initiating treatment, and no studies have identified specific biomarkers that can definitively diagnose sepsis in burn patients. Burn wounds that are not initially excised are initially colonized by gram-positive organisms; however, over time, the microbial composition shifts to gram-negative bacteria, yeasts, molds, and eventually multidrug-resistant pathogens. Due to these dynamic changes in wound flora, it is preferable to assess the wound for clinical signs of infection before obtaining cultures, as burn wounds are invariably colonized. Conducting a wound culture in the absence of infection signs may lead to inappropriate treatment.
Whenever possible, wound cultures should be obtained before initiating antibiotic therapy if signs or symptoms of infection are present. However, routine wound cultures are not recommended in the absence of infection indicators, as bacterial presence alone is not diagnostic. Surface swabbing has low diagnostic specificity, and the primary method for detecting infection remains the identification of significant changes in wound appearance. This is a weak recommendation based on very low-quality evidence.48
In 2024, an expert consensus43 evaluated recommendations on antibiotic use in burn patients, involving organizations such as the Global Alliance for Infection in Surgery, the Surgical Infection Society Europe, the World Surgical Infection Society, the American Association for the Surgery of Trauma, and the World Society of Emergency Surgery. These groups emphasize that routine systemic antibiotic prophylaxis is not recommended for burn patients, except in specific circumstances.
They recommend its use to prevent infections in skin grafts or in patients undergoing intubation and mechanical ventilation, preferably administering them before intubation and adjusting them based on the pharmacokinetics of the antibiotic. Additionally, they emphasize the importance of proper source control, such as extensive irrigation and removal of contaminated material, to reduce the risk of infection without fostering antimicrobial resistance.43
Therapeutic Strategies
Antibiotic therapy should be tailored to the pathogen’s susceptibility and the patient’s pharmacokinetics. The selection of antibiotics should be guided by regional resistance patterns and, whenever feasible, tailored to the results of pathogen-specific susceptibility testing, specific institutional resistance patterns, enabling identification of resistance prevalence and subsequently guiding treatment according to the patient’s specific cultures.43 It is essential to identify the infecting organism to administer the appropriate treatment. Some studies have documented a high incidence of negative cultures in burn patients with sepsis, reaching up to 52.6%. Therefore, clinical findings should not be disregarded even when cultures yield negative results.48
Additional Considerations
Adjustment according to culture and antibiogram: Therapy should be modified based on microbiological results and the clinical progression of the patient.
Therapeutic monitoring: Monitoring of vancomycin levels in critically ill patients and dose adjustments in cases of renal insufficiency.
Infection prevention and control: Strict adherence to protocols for managing invasive devices, removing them if they are not necessary for management, and maintaining strict isolation measures.
The burn wound should be evaluated within one hour of diagnosis, and if there are indications of invasive infection, prompt excision should be performed, with cultures obtained to guide antibiotic therapy. Empirical broad-spectrum antibiotics should ideally be initiated within one hour in cases of septic shock and within three hours for sepsis. This is a weak recommendation based on very low-level evidence.
For comprehensive management, behaviors have been extrapolated from the Surviving Sepsis Campaign that, in theory, should improve outcomes in burn patients with sepsis. This recommendation is the first step in providing measurable resuscitation goals to validate them or at least develop better goals for burn patients with sepsis.
1. Obtain serum lactate levels and base deficit to guide response to management.
2. Obtain adequate intravenous (IV) access.
3. Insert a Foley catheter for strict urine monitoring.
4. Insert an arterial line to monitor blood pressure and arterial blood gases.
5. Administer an appropriate fluid bolus if hypotension or reduced urine output is present.
6. If mean arterial pressure (MAP) is < 65 mm Hg, initiate vasopressors and establish invasive hemodynamic monitoring.
7. The use of an inotropic agent should be considered if there are signs of heart failure and other causes of shock, such as hypovolemia, have been excluded.
8. Start broad-spectrum antibiotics (after obtaining cultures, use direct antimicrobial therapy).
9. Obtain source control.
10. Prevent hypothermia (< 35° C).
Strong recommendation, very low-level evidence.48
Management of burn patients with deep infection should be addressed comprehensively in a specialized burn unit where surgical care, hemodynamic support, and proper infection identification and management can be provided. It is essential to apply the Surviving Sepsis Campaign goals to complement clinical suspicion and optimize the body’s response, minimizing organ dysfunction. The patient’s evolution will be variable, depending on factors such as the extent of burns, treatment response, and associated complications. From the perspective of the general surgeon, early stabilization, control of the infection focus with timely surgical debridement, and infection prevention should be prioritized, always adjusting treatment according to the patient’s clinical response.
Conclusion
The present document establishes a clear guideline based on the most recent scientific evidence available for the diagnosis and management of invasive wound infections in burn patients at the Hospital Universitario del Valle, Cali- Colombia. Through a structured approach, the aim is to standardize practices that prioritize early diagnosis through defined clinical criteria, based on changes in the physical aspects of the wound, supported by signs of systemic inflammation and alterations in laboratory parameters specific to this population. Additionally, it promotes the selection of initial antibiotics based on the local epidemiological and resistance profile, always adjusting treatment according to microbiological findings and the clinical progression of the patient.
The implementation of these guidelines will not only optimize the care of burn patients but also reduce complications, improve resource efficiency, and establish an integrated management model that can serve as a reference for other institutions (►Fig. 7).
REFERENCES
1. World Health Organization. Burns. [Internet]. Geneva: World Health Organization; 2024 Dec 13 [cited 2024 Dec 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/burns
2. Kiley JL, Greenhalgh DG. Infections in Burn Patients. Surg Clin North Am 2023;103(03):427-437. Doi: 10.1016/j.suc.2023.02.005
3. Wen ZL, Zhang LD, Liu SZ, Liu J, Chen YZ, Chen DC. Effect of broadspectrum antibiotics on bacterial translocation in burned or septic rats. Chin Med J (Engl) 2019;132(10):1179-1187. Doi: 10.1097/CM9.0000000000000242
4. Chen LW, Chen PH, Fung CP, Hsu CM. Dead bacteria reverse antibioticinduced host defense impairment in burns. J Am Coll Surg 2014;219 (04):606-619. Doi: 10.1016/j.jamcollsurg.2014.04.016
5. Vickers ML, Malacova E, Milinovich GJ, Harris P, Eriksson L, Dulhunty JM, Cotta MO. Modifiable risk factors for multidrugresistant Gram-negative infection in critically ill burn patients: a systematic review and meta-analysis. ANZ J Surg 2019;89(10): 1256-1260. Doi: 10.1111/ans.15393
6. Sturtevant AB, Cassell GH, Bobo RA, Feary TW. Effect of antibiotic treatment on the incidence of infectious drug resistance among intestinal lactose-fermenting bacteria isolated from burn patients. Infect Immun 1971;3(03):411-415. Doi: 10.1128/iai.3.3.411-415.1971
7. Bamberg M, Menger MM, Thiel JT, Lauer H, Viergutz T, Fontana J. Antibiotics in patients with severe burn injury-A modifiable variable in hypernatremia etiology. Injury 2024;55(09):111573. Doi: 10.1016/j.injury.2024.111573
8. Ferrada R. Manejo de quemaduras: Básico y avanzado. Bogotá, Colombia: Grupo Distribuna; 2015
9. Ozlu O, Basaran A. Infections in Patients With Major Burns: A Retrospective Study of a Burn Intensive Care Unit. J Burn Care Res 2022;43(04):926-930. Doi: 10.1093/jbcr/irab222
10. Belba MK, Petrela EY, Belba AG. Epidemiology and outcome analysis of sepsis and organ dysfunction/failure after burns. Burns 2017;43(06):1335-1347. Doi: 10.1016/j.burns.2017.02.017
11. Posluszny JA Jr, Conrad P, Halerz M, Shankar R, Gamelli RL. Surgical burn wound infections and their clinical implications. J Burn Care Res 2011;32(02):324-333. Doi: 10.1097/BCR.0b013e31820aaffe
12. Richards C, Emori TG, Edwards J, Fridkin S, Tolson J, Gaynes R. Characteristics of hospitals and infection control professionals participating in the National Nosocomial Infections Surveillance System 1999. Am J Infect Control 2001;29(06):400-403. Doi: 10.1067/mic.2001.118408
13. Fitzwater J, Purdue GF, Hunt JL, O’Keefe GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma 2003;54(05):959-966. Doi: 10.1097/01.TA.0000029382.26295.AB
14. Mason AD Jr, McManus AT, Pruitt BA Jr. Association of burn mortality and bacteremia. A 25-year review. Arch Surg 1986; 121(09):1027-1031. Doi: 10.1001/archsurg.1986.01400090057009
15. Ramirez-Blanco CE, Ramirez-Rivero CE, Diaz-Martinez LA, Sosa- Avila LM. Infection in burn patients in a referral center in Colombia. Burns 2017;43(03):642-653. Doi: 10.1016/j.burns.2016.07.008
16. Teller P, White TK. The physiology of wound healing: injury through maturation. Surg Clin North Am 2009;89(03):599-610. Doi: 10.1016/j.suc.2009.03.006
17. Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 10th ed. Elsevier; 2021
18. D’Arpa P, Leung KP. Toll-Like Receptor Signaling in Burn Wound Healing and Scarring. Adv Wound Care (New Rochelle) 2017;6 (10):330-343. Doi: 10.1089/wound.2017.0733
19. Tompkins RG. Genomics of injury: The Glue Grant experience. J Trauma Acute Care Surg 2015;78(04):671-686. Doi: 10.1097/TA.0000000000000568
20. Cundell AM. Microbial ecology of the human skin. Microb Ecol 2018;76(01):113-120. Doi: 10.1007/800248-016-0789-6
21. Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, et al; NISC Comparative Sequencing Program. A diversity profile of the human skin microbiota. Genome Res 2008;18(07): 1043-1050. Doi: 10.1101/gr.075549.107
22. Lange-Asschenfeldt B, Marenbach D, Lang C, Patzelt A, Ulrich M, Maltusch A, et al. Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Skin Pharmacol Physiol 2011; 24(06):305-311. Doi: 10.1159/000328728
23. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 2006;19(02):403-434. Doi: 10.1128/CMR.19.2.403-434.2006
24. Goff DA, Kullar R, Goldstein EJC, Gilchrist M, Nathwani D, Cheng AC, et al. A global call from five countries to collaborate in antibiotic stewardship: united we succeed, divided we might fail. Lancet Infect Dis 2017;17(02):e56-e63. Doi: 10.1016/S1473-3099(16)30386-3
25. A Global Declaration on Appropriate Use of Antimicrobial Agents across the Surgical Pathway. Surg Infect (Larchmt) 2017;18(08): 846-853. Doi: 10.1089/sur.2017.219
26. Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg 2009;36(04):583-596. Doi: 10.1016/j.cps.2009.05.001
27. Kennedy P, Brammah S, Wills E. Burns, biofilm and a new appraisal of burn wound sepsis. Burns 2010;36(01):49-56. Doi: 10.1016/j.burns.2009.02.017
28. Yin S, Jiang B, Huang G, Gong Y, You B, Yang Z, et al. Burn Serum Increases Staphylococcus aureus Biofilm Formation via Oxidative Stress. Front Microbiol 2017;8:1191. Doi:10.3389/fmicb.2017.01191
29. Patterson EK, Cepinskas G, Fraser DD. Endothelial Glycocalyx Degradation in Critical Illness and Injury. Front Med (Lausanne) 2022;9:898592. Doi: 10.3389/fmed.2022.898592
30. Tapking C, Hernekamp JF, Horter J, Kneser U, Haug V, Vogelpohl J, et al. Influence of burn severity on endothelial glycocalyx shedding following thermal trauma: A prospective observational study. Burns 2021;47(03):621-627. Doi: 10.1016/j.burns.2020.07.021
31. Osuka A, Kusuki H, Yoneda K, Matsuura H, Matsumoto H, Ogura H, Ueyama M. Glycocalyx Shedding is Enhanced by Age and Correlates with Increased Fluid Requirement in Patients with Major Burns. Shock 2018;50(01):60-65. Doi: 10.1097/SHK.0000000000001028
32. Ladhani HA, Young BT, Posillico SE, Yowler CJ, Brandt CP, Claridge JA, Khandelwal AK. Risk Factors for Wound Infection in Outpatients With Lower Extremity Burns. Am Surg 2021;87(07): 1118-1125. Doi: 10.1177/0003134820952387
33. Bourgi J, Said JM, Yaakoub C, Atallah B, Al Akkary N, Sleiman Z, Ghanimé G Bacterial infection profile and predictors among patients admitted to a burn care center: A retrospective study. Burns 2020;46(08):1968-1976. Doi: 10.1016/j.burns.2020.05.004
34. Strassle PD, Williams FN, Weber DJ, Sickbert-Bennett EE, Lachiewicz AM, Napravnik S, et al. Risk Factors for Healthcare-Associated Infections in Adult Burn Patients. Infect Control Hosp Epidemiol 2017;38(12):1441-1448. Doi: 10.1017/ice.2017.220
35. Karaaslan A, Çetin C, Köle MT, Dereli M, Tekol SD, Filinte G, Akin Y. Infections in Pediatric Patients With Burn Injury: 6 Years of Experience. Pediatr Infect Dis J 2023;42(01):8-12. Doi: 10.1097/INF.0000000000003741
36. Fochtmann-Frana A, Freystätter C, Vorstandlechner V, Barth A, Bolliger M, Presterl E, et al. Incidence of risk factors for bloodstream infections in patients with major burns receiving intensive care: A retrospective single-center cohort study. Burns 2018;44 (04):784-792. Doi: 10.1016/j.burns.2017.12.009
37. Pereira CT, Barrow RE, Sterns AM, Hawkins HK, Kimbrough CW, Jeschke MG, et al. Age-dependent differences in survival after severe burns: a unicentric review of 1,674 patients and 179 autopsies over 15 years. J Am Coll Surg 2006;202(03):536-548. Doi: 10.1016/j.jamcollsurg.2005.11.002
38. Yan J, Hill WF, Rehou S, Pinto R, Shahrokhi S, Jeschke MG. Sepsis criteria versus clinical diagnosis of sepsis in burn patients: A validation of current sepsis scores. Surgery 2018;164(06):1241-1245. Doi: 10.1016/j.surg.2018.05.053
39. Greenhalgh DG, Saffle JR, Holmes JH IV, Gamelli RL, Palmieri TL, Horton JW, et al; American Burn Association Consensus Conference on Burn Sepsis and Infection Group. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 2007;28(06):776-790. Doi: 10.1097/BCR.0b013e3181599bc9
40. George A, Bang RL, Lari AR, Gang RK. Acute thrombocytopenic crisis following burns complicated by staphylococcal septicaemia. Burns 2001;27(01):84-88. Doi: 10.1016/s0305-4179(00)00065-6
41. Ferrada R, Aragón N, Becerra C. Cultivo biopsia en quemaduras. Rev Colomb Cir 1992;7(03):151-153
42. Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 2018;67(06):e1-e94. Doi: 10.1093/cid/ciy381
43. Coccolini F, Sartelli M, Sawyer R, Rasa K, Ceresoli M, Viaggi B, et al. Antibiotic prophylaxis in trauma: Global Alliance for Infection in Surgery, Surgical Infection Society Europe, World Surgical Infection Society, American Association for the Surgery of Trauma, and World Society of Emergency Surgery guidelines. J Trauma Acute Care Surg 2024;96(04):674-682. Doi: 10.1097/TA.0000000000004233
44. Li AT, Moussa A, Gus E, Paul E, Yii E, Romero L, et al. Biomarkers for the Early Diagnosis of Sepsis in Burns: Systematic Review and Meta-analysis. Ann Surg 2022;275(04):654-662. Doi: 10.1097/sla.0000000000005198
45. Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med 1998;24(08):888-889. Doi: 10.1007/s001340050683
46. Cabral L, Afreixo V, Almeida L, Paiva JA. The Use of Procalcitonin (PCT) for Diagnosis of Sepsis in Burn Patients: A Meta-Analysis. PLoS One 2016;11(12):e0168475. Doi: 10.1371/journal.pone.0168475
47. Zhang YJ, Guo ZH, Ming ZG, Hao ZM, Duan P. Meta-analysis of the diagnostic value of serum procalcitonin for burn sepsis in adults. Eur Rev Med Pharmacol Sci 2023;27(15):7188-7200. Doi: 10.26355/eurrev_202308_33292
48. Greenhalgh DG, Hill DM, Burmeister DM, Gus EI, Cleland H, Padiglione A, et al. Surviving Sepsis After Burn Campaign. Burns 2023;49(07):1487-1524. Doi: 10.1016/j.burns.2023.05.003
1. Department of General Surgery, School of Medicine, Universidad del Valle, Cali,Valle
del Cauca,Colombia
2. School of Medicine, Universidad Nacional de Colombia, Bogotá D.C., Colombia
Approval from the Research Ethics Committee
This article is a review, so it did not include patients and was not conducted at a specific healthcare institution. We understand that, according to the journal’s requirements, a letter from the ethics committee is not necessary. We remain available for any additional requests.
Address for correspondence Juan Paulo Benitez, Departamento de Cirugía General, Facultad de Medicina, Universidad del Valle, Calle 4B #36-00, Cali, Valle del Cauca, Colombia, (e-mail: benitez.juan@correounivalle.edu.co).
Article received: January 20, 2025.
Article accepted: March 24, 2025.
Conflict of Interests
The authors have no conflict of interests to declare.







 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter