

Original Article - Year 2011 - Volume 26 -
Effects of hyperbaric oxygenation and N-acetylcysteine on the survival of random-pattern skin flaps in rats
Efeito da oxigenação hiperbárica e da N-acetilcisteína na viabilidade de retalhos cutâneos em ratos
ABSTRACT
BACKGROUND: Advances in plastic surgery techniques have enabled reconstruction of extensive wound damage, especially through the use of random flaps. However, the limiting factor for the use of these flaps is the unpredictable blood supply, which may produce irreversible damage to the microcirculation and result in partial or complete flap necrosis, making the wound more susceptible to infection. Therefore, improvement of random flaps, especially in the distal extremity, has been an essential goal for the success of this technique. The objective of this study was to investigate the effects of hyperbaric oxygenation (HBO), N-acetylcysteine (NAC), and the combination of both (HBO + NAC) on the degree of necrosis in modified McFarlane random skin flaps on Wistar rats.
METHODS: A total of 32 male Wistar rats were randomly divided into a sham treatment group (SG, n = 8), N-acetylcysteine group (NACG, n = 8), hyperbaric oxygenation group (HBOG, n = 8), and hyperbaric oxygenation plus N-acetylcysteine group (HNG, n = 8). Modified McFarlane random flaps were created in the dorsal region of the rats.
RESULTS: The average area of the flaps exhibiting necrosis was 18.3%, 24.3%, 12.6%, and 14.9%, in the SG, NACG, HBOG, and HNG, respectively. The necrotic areas in the HBOG and HNG were significantly smaller than that in the NACG.
CONCLUSIONS: HBO treatment was associated with a reduction in the area of necrosis in the skin flaps. NAC treatment alone gave poor results. The use of HBO and NAC in combination did not improve the outcome compared with the use of HBO alone. The findings suggest that oxygen diffusion through the interstitial space was the factor responsible for the favorable results of HBO.
Keywords: Hyperbaric oxygenation. N-acetylcysteine. Surgical flaps.
RESUMO
INTRODUÇÃO: Os avanços das técnicas em cirurgia plástica permitiram a reconstrução de extensos defeitos causados por ferimentos, entre as quais destaca-se a utilização dos retalhos randômicos. No entanto, o fator limitante para a utilização desses retalhos é a imprevisibilidade de sua vascularização distal, o que poderá ocasionar danos irreversíveis à microcirculação, resultando em necrose parcial ou completa do retalho, tornando a ferida mais suscetível a infecção. Portanto, melhorar a viabilidade do retalho randômico, principalmente em sua extremidade distal, tem sido uma meta importante para o sucesso dessa técnica. O objetivo deste estudo foi investigar o papel da oxigenação hiperbárica (OHB), da N-acetilcisteína (NAC) e da associação de ambas (OHB + NAC) na área de necrose em retalhos randômicos modificados de McFarlane em pele de ratos Wistar.
MÉTODO: No total, 32 ratos Wistar machos foram divididos aleatoriamente em grupo Sham (GS, n = 8), grupo N-acetilcisteína (GNAC, n = 8), grupo oxigênio hiperbárico (GOHB, n = 8) e grupo oxigênio hiperbárico + N-acetilcisteína (GHN, n = 8). Sob anestesia geral, foi executado um retalho randômico modificado de McFarlane na região dorsal dos ratos.
RESULTADOS: A necrose média foi de 18,3%, 24,3%, 12,6% e 14,9%, respectivamente, nos grupos GS, GNAC, GOHB e GHN. Os grupos GOHB e GHN apresentaram diferença significativa quando comparados ao grupo GNAC.
CONCLUSÕES: A OHB está associada a redução da área de necrose do retalho cutâneo. A NAC foi associada a maus resultados quando usada isoladamente. A associação dos dois procedimentos, OHB e NAC, não potencializou os resultados favoráveis observados com o uso da OHB isoladamente. As descobertas sugerem que a difusão de oxigênio através do espaço intersticial foi o fator determinante de resultados mais favoráveis da OHB.
Palavras-chave: Oxigenação hiperbárica. N-acetilcisteína. Retalhos cirúrgicos.
Advances in plastic surgery techniques have enabled reconstruction of extensive wound damage, especially through the use of random flaps. However, the limiting factor for use of these flaps is the unpredictable distal blood supply, which may produce irreversible damage to the microcirculation and result in partial or complete flap necrosis, making the wound more susceptible to infection. Therefore, improvement of random flaps, especially in the distal extremity, has been an essential goal for the success of this technique1-3.
The cell damage that occurs during tissue reperfusion after ischemia results from a series of events involving oxygen free radical production and release of inflammatory mediators4,5. Molecular oxygen therefore plays an important role in the healing process4. Hyperoxia caused by hyperbaric oxygenation (HBO) increases tissue tolerance to ischemia2 and increases biological antioxidant defense mechanisms6,7. HBO has a protective effect on the microcirculation, possibly by interfering with the deleterious effects of activated neutrophils on the microvascular endothelium8. In turn, inhibition of neutrophil function stimulates angiogenesis and increases fibroblast activity and collagen synthesis4,9.
Mammals have a complex antioxidant system to protect cells from stress. One of the most important components of the intracellular antioxidant system is glutathione, a powerful free radical scavenger that is depleted during ischemia-reperfusion injury10. N-acetylcysteine (NAC) is a prodrug that provides bioavailable cysteine for glutathione replacement9 when cellular glutathione levels are reduced by the presence of reactive oxygen species (ROS). NAC prevents many of the deleterious effects of oxidative stress during exposure to HBO11. The objective of this study was to investigate the role of HBO and NAC, either alone or in combination, on the viability of random flaps in rats, using the model proposed by McFarlane et al.12.
METHODS
Ethical Aspects
The experimental protocol was approved by the Ethics Committee of Universidade Federal de São Paulo (UNIFESP; number 1431-1403). All procedures strictly followed the existing regulations on animal experimentation of the Brazilian Association of Animal Experimentation (COBEA).
Animal Care and Experimental Groups
Thirty-two male Wistar rats weighing between 280 g and 300 g were kept in individual cages in acoustically isolated rooms at 25°C. The rooms had artificial light, and the animals had access to food and water ad libitum. The animals were randomly divided into 4 groups: sham group (SG; n = 8), N-acetylcysteine group (NACG; n = 8), hyperbaric oxygenation group (HBOG; n = 8), and hyperbaric oxygenation + NAC (HNG; n = 8).
Anesthetic Procedure
Animals were deprived of solid food for 6 hours and liquids for 4 hours, and were then anesthetized with 5 mg/kg of intramuscular acepromazine (Acepran® 0.2%, Vetnil Indústria e Comércio de Produtos Veterinários Ltda. São Paulo, Brazil). Ten minutes later, they received a combination of 50 mg/kg of intramuscular ketamine (Ketalar®, Pfizer do Brasil, São Paulo, Brazil) and 10 mg/kg of intramuscular xylazine (Rompum®, Bayer, São Paulo, Brazil).
Surgical Procedure
Under general anesthesia, the dorsal regions were epilated and the animals were fixed in the prone position. A rectangular area of skin (2 cm x 8 cm) was marked with ink, with the base at the 7th cervical vertebra towards the caudal position, setting the spine as the central mark. The markings were incised with a Nº 15 scalpel, and the skin flap was detached from the dorsal muscles (Figure 1). A polyethylene film was placed on the muscular portion to cover the whole area of the wound, thus acting as a barrier between the skin flap and muscle tissue (Figure 2). The skin flap was then sutured into its original position using an interrupted suture with 3.0 nylon (Mononylon®, Ethicon, São Paulo, Brazil).
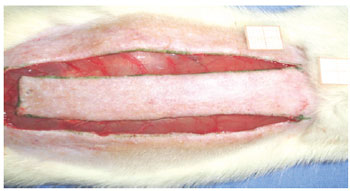
Figure 1 - Standardized random McFarlane skin flap (2 cm x 8 cm), with the base at the 7th cervical vertebra.
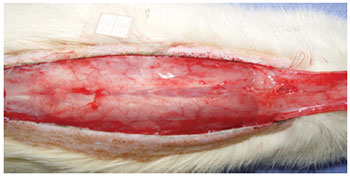
Figure 2 - Polyethylene film placed on the muscle layer, which covers the entire wound area and acts as a barrier between the skin and muscles.
Procedure for Administration of NAC and Distilled Water
Animals in the NACG and HNG received a dose of 300 mg/kg of NAC (Fluimucil® acetylcysteine 300 mg/3 ml, Zambon Laboratório Farmacêutico Ltda., São Paulo, Brazil) by intraperitoneal injection immediately after creating the skin flap and then every 24 hours for 7 days. The SG and HBOG received 1 ml of distilled water (Isofarma, São Paulo, Brazil) by the same route following the same schedule.
Procedure with HBO
HBO was performed in an experimental hyperbaric chamber for animals13 at the Universidade Regional do Alto Uruguai Campus Erechim (URI) (Figure 3). Before pressurization, the chamber was washed for 5 minutes with 100% medicinal oxygen. The oxygen pressure was then increased at a constant rate until it reached 2.4 ATA. The oxygen concentration was monitored with a calibrated oximeter. The animals were randomly placed in the hyperbaric chamber. Animals in the HBOG and HNG were exposed to 100% oxygen at 2.4 ATA for 2 hours starting 15 minutes after flap fixation, and then every 24 hours for 7 days.

Figure 3 - Hyperbaric chamber for hyperbaric oxygenation of animals.
Daily Procedures
The sequence of procedures for each group is summarized in Figure 4. The animals were randomly assigned to the following groups: SG (n = 8), received distilled water injections once daily for 7 days; NACG (n = 8), received 300 mg/kg NAC once daily for 7 days; HBOG (n = 8), exposed to 100% hyperbaric oxygen at 2.4 ATA for 2 hours daily for 7 days; and HNG (n = 8), received both the NAC injection and the hyperbaric oxygen treatments daily for 7 days.
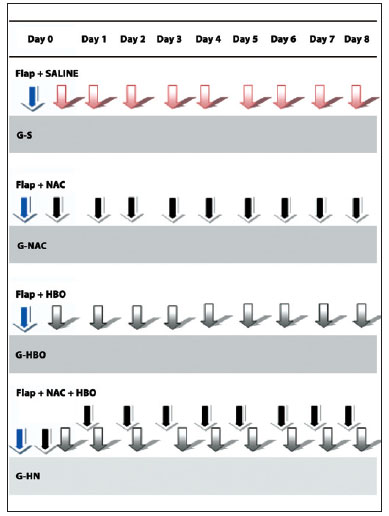
Figure 4 - Procedure flowchart for different groups: SG, creation of the flap and 8 daily intraperitoneal injections of saline; NACG, creation of the flap and 8 daily intraperitoneal injections of N-acetylcysteine; HBOG, creation of the flap and 8 daily sessions of hyperbaric oxygenation; HNG, creation of the flap and 8 daily intraperitoneal injections of N-acetylcysteine followed by hyperbaric oxygenation. HNG = hyperbaric oxygen group + N-acetylcysteine; NACG = N-acetylcysteine group; HBOG = hyperbaric oxygen group; SG = sham group.
Follow-up
The animals were examined twice daily for signs of infection at the incision site or fever. If any sign of severe suffering was identified, the veterinarian stopped the investigation and the animals were euthanized.
On the 8th postoperative day, the animals were anesthetized and fixed in the prone position. The dorsal region was photographed from a standard distance using a 7.2 megapixel digital camera (SonyTP200, Sony, Japan), and the files were saved in JPEG format.
Euthanasia
After the animals had been anesthetized and the skin flaps removed, the animals were euthanized by placing them in a chamber with carbon dioxide (CO2) until the animals went into cardiac arrest.
Determination of the Necrosis Area of the Flap
On the 8th postoperative day, the area of the flap was photographed and compared with the appearance on the first day of the experiment. Necrosis was defined by the formation of a dark color and scabs. The average necrotic area was determined for all groups. Photographic images were captured using Image Pro Plus 4.5® software. All results were represented as average and standard deviation.
Statistical Analysis
The results are expressed as the percentage area of the skin flap identified as necrotic, and are presented as the average and standard deviation (SPSS version 11.0). Differences between the average necrotic areas were compared by variance analysis (ANOVA) followed by the Bonferroni post hoc test. A P value of 5% (P < 0.05) was considered statistically significant.
RESULTS
Figure 5 shows the average percentage area of necrosis according to the treatment group. The average areas of necrosis were 18.3% in SG, 24.3% in NACG, 12.6% in HBOG, and 14.9% in HNG.
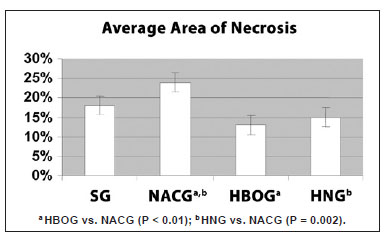
Figure 5 - Average and standard deviation of the percentage area of necrosis of the random skin flaps in the SG, NACG, HBOG, and HNG. There were significant differences between the HBOG and NACG (P < 0.01), and between the HNG and NACG (P < 0.001) (ANOVA). HNG = hyperbaric oxygen group + N-acetylcysteine; NACG = N-acetylcysteine group; HBOG = hyperbaric oxygen group; SG = sham group.
Table 1 describes the results of the macroscopic assessment (area in mm2) for each treatment group.
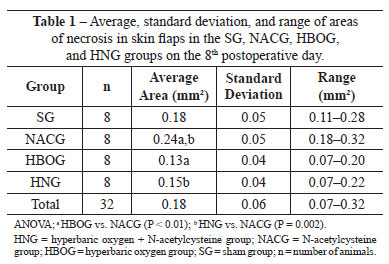
The HBOG showed a smaller area of necrosis than the SG, but the difference was not significant (P = 0.12). The viability of the HBOG flaps was significantly better than that of the NACG (P < 0.01), and was approximately the same as the HNG. The area of necrosis significantly differed between the HNG and NACG (P < 0.01).
In this study, HBO alone increased the viability of the skin flaps. Unexpectedly, the combination treatment of NAC plus HBO did not improve flap viability compared to the effect of HBO alone.
DISCUSSION
There is controversy surrounding the angiogenic properties of HBO. HBO has been used in the treatment of skin wounds to increase the tension of fibers and to stimulate angiogenesis2,3. The HBO-induced angiogenic effect is responsible for high oxygen tension, which may persist for some hours after HBO6,8. Repeated exposure to HBO produced the on-off effect, providing a favorable environment in the flaps of the HBO groups compared to the group that did not use HBO (SG and NACG). In this study, HBO administration for 2 hours per day for 7 days improved the viability of random skin flaps, in sharp contrast to the original hypothesis that suggested that the exposure to additional oxygen would increase the production of free radicals and increase the area of necrosis in the flap. The most accepted current hypothesis suggests that HBO would cause peripheral arteriolar vasoconstriction, which would theoretically neutralize the reflex post-ischemia vasodilation, thereby reducing the interstitial fluid and edema, and improving the viability of the flap3,7. Hong et al.14 demonstrated that hypoxic tissues subjected to HBO develop increased plasma oxygen pressure (pO2), which reduces tissue hypoxia, likely due to the increased rate of oxygen diffusion.
NAC is a precursor of glutathione, a potent endogenous antioxidant that acts by inhibiting the induction of pro-inflammatory cytokines, nitric oxide synthase (iNOS), ICAM-1, and VCAM-110,15-17, in addition to stimulating the production of nitric oxide (NO)18. In this study, flaps treated only with HBO showed a similar increase in viability when compared to flaps treated with the combination of HBO and NAC, suggesting that these agents do not have additive effects. The results were the poorest in groups treated with distilled water or NAC alone. The dose of NAC (300 mg/kg/day) used in this study was selected based on the low toxicity of the drug and on the favorable results obtained for the protection of random skin flaps in rats in other studies. The plastic barrier interposed between the flap and the tissue bed prevented revascularization of the flap19.
The use of HBO in combination with NAC may have provided protection from the deleterious effects of NAC. When used alone, NAC resulted in a larger area of necrosis (24%) as compared to the area of necrosis produced by the combination of HBO and NAC (15%) (P < 0.01). It is likely that, in this study, the high concentration of NAC inhibited angiogenesis and wound healing, although it is not known if this was due to an imbalance in the redox state or through another mechanism9. It has been shown that HBO can increase tissue tolerance to ischemia, and reduce subsequent metabolic disturbances. HBO also improves tissue microcirculation by reducing platelet aggregation. These characteristics, combined with the increased capacity of plasma to transport dissolved oxygen to areas inaccessible to red blood cells, have been shown to be an important mechanism underlying the beneficial effect of oxygenation in several hypoxic tissues2,20.
In this study, HBO combined with antioxidants did not improve the survival of flaps compared to the use of HBO alone, suggesting that the potential toxic effects of hyperoxia, such as production of ROS, were not neutralized by antioxidant therapy with NAC. It is believed that low concentrations of ROS may play a beneficial role in tissue healing6. Oxidizing species such as free radicals and hydrogen peroxide may serve as cellular messengers to mediate processes such as extracellular matrix formation, the action of cytokines, angiogenesis, and cellular mobility, and in doing so stimulate the healing process9.
CONCLUSIONS
Treatment with HBO reduced necrosis in skin flaps, whereas NAC treatment alone gave poor results. The combination of HBO and NAC did not improve the outcome compared with HBO treatment alone. The findings suggest that the favorable effects of HBO could be attributed to oxygen diffusion through the interstitial space.
REFERENCES
1. Matsumara H, Yoshizawa N, Vedder NB, Watanabe K. Preconditioning of the distal portion of a rat random-pattern skin flap. Br J Plast Surg. 2001;54(1):58-61.
2. Richards L, Lineaweaver WC, Stile F, Zhang F, Zhang F. Effect of hyperbaric oxygen therapy on the tubed pedicle flap survival in a rat model. Ann Plast Surg. 2003;50(1):51-6.
3. Ulkür E, Yüksel F, Açikel C, Celiköz B. Effect of hyperbaric oxygen on pedicle flaps with compromised circulation. Microsurgery. 2002;22(1):16-20.
4. Buras J. Basic mechanisms of hyperbaric oxygen in the treatment of ischemic-reperfusion injury. Int Anesthesiol Clin. 2000;38(1):91-109.
5. Ayhan S, Tugay C, Norton S, Araneo B, Siemionow M. Dehydroepiandrosterone protects the microcirculation of the muscle flaps from ischemia-reperfusion injury by reducing the expression of adhesion molecules. Plast Reconstr Surg. 2003;111(7):2286-94.
6. Zhang T, Gong W, Li Z, Yang S, Zhang K, Yin D, et al. Efficacy of hyperbaric oxygen on survival of random pattern skin flap in diabetic rats. Undersea Hyperb Med. 2007;34(5):335-9.
7. Ulkür E, Karagoz H, Ergun O, Celikoz B, Yildiz S, Yildirim S. The effect of hyperbaric oxygen therapy on the delay procedure. Plast Reconstr Surg. 2007;119(1):86-94.
8. Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Library. 2004;(4). Disponível em: http//www.cochrane.org/reviews/ (acesso em 26/1/2010).
9. Kunnavatana SS, Quan SY, Koch RJ. Combined effect of hyperbaric oxygen and N-acetylcysteine on fibroblast proliferation. Arch Otolaryngol Head Neck Surg. 2005;131(9):809-14.
10. Glantzounis GK, Yang W, Koti RS, Mikhailidis DP, Seifalian AM, Davidson BR. Continuous infusion of N-acetylcysteine reduces liver warm ischaemia reperfusion injury. Br J Surg. 2004;91(10):1330-9.
11. Arrais-Silva WW, Colhone MC, Ayres DC, Souza Souto PC, Giorgio S. Effects of hyperbaric oxygen on Leishmania amazonensis promastigotes and amastigotes. Parasitol Int. 2005;54(1):1-7.
12. McFarlane RM, Deyoung G, Henry RA. The design of a pedicle flap in the rat to study necrosis and its prevention. Plast Reconstr Surg. 1965;35:177-82.
13. Rech FV, Fagundes DJ, Hermanson R, Rivoire HC, Fagundes ALN. Uma proposta de câmara hiperbárica para uso em animal de experimentação e uso veterinário. Acta Cir Bras. 2008;23(4):384-90.
14. Hong JP, Kwon H, Chung YK, Jung SH. The effect of hyperbaric oxygen on ischemia-reperfusion injury: an experimental study in a rat musculocutaneous flap. Ann Plast Surg. 2003;51(5):478-87.
15. Haase M, Haase-Fielitz A, Ratnaike S, Reade MC, Bagshaw SM, Morgera S, et al. N-Acetylcysteine does not artifactually lower plasma creatinine concentration. Nephrol Dial Transplant. 2008;23(5):1581-7.
16. Adabag AS, Ishani A, Koneswaran S, Johnson DJ, Kelly RF, Ward HB, et al. Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. Am Heart J. 2008;155(6):1143-9.
17. Miner SE, Dzavik V, Nguyen-Ho P, Richardson R, Mitchell J, Atchison D, et al. N-acetylcysteine reduces contrast-associated nephropathy but not clinical events during long-term follow-up. Am Heart J. 2004;148(4):690-5.
18. Deshpande VS, Kehrer JP. Mechanisms of N-acetylcysteine-driven enhancement of MK886-induced apoptosis. Cell Biol Toxicol. 2006;22(4):303-11.
19. Abla LE, Gomes HC, Percario S, Ferreira LM. Acetylcysteine in random skin flap in rats. Acta Cir Bras. 2005;20(2):121-3.
20. Kiumehr S, Demehri S, Rabbani S, Amanpour S, Mohagheghi MA, Dehpour AR. Preconditioning of the rat random-pattern skin flap: modulation by opioids. Br J Plast Surg. 2005;58(1):58-64.
1. Doctor, Associate Member of the Brazilian Association of Plastic Surgery, Professor at the Department of Surgery of Universidade Católica de Pelotas, Pelotas, RS, Brazil.
2. Doctor, Associate Professor in Surgical Technique and Experimental Surgery at the Department of Surgery of Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil.
3. Medical Student, President of the Academic League of Plastic Surgery of Universidade Federal de Pelotas, Pelotas, RS, Brazil.
4. Medical Student at Universidade Federal de Pelotas, Pelotas, RS, Brazil.
Correspondence to:
Fernando Passos da Rocha
Praça Piratinino de Almeida, 13 - Centro
Pelotas, RS, Brazil - CEP 96015-290
E-mail: artigosplastica@hotmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Paper received: June 21, 2011
Paper accepted: July 29, 2011
Study conducted at Universidade Federal de Pelotas and at Universidade Católica de Pelotas, Pelotas, RS, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter