

Original Article - Year 2014 - Volume 29 -
Therapeutic approTherapeutic Approach To The Parry-Romberg Syndrome Based On A Severity Grading System
Abordagem terapêutica da síndrome de parry-romberg baseada em um sistema de classificação de gravidade
ABSTRACT
INTRODUCTION: The Parry-Romberg Syndrome (PRS) is characterized by progressive hemifacial atrophy that often leads to severe esthetic and functional difficulties. Although there are systems for grading disease severity, none have proven ideal in optimizing the therapeutic approach to these patients. This study aimed to establish the surgical strategies for the treatment of PRS based on a new system for severity grading of the disease.
METHODS: This retrospective study included PRS patients undergoing surgery between 2005 and 2011. The surgical strategies were adapted for each patient according to a clinical severity grading system based on disease progression: type I, affecting the epidermis, dermis, and subcutaneous tissue; type II, type I + muscle involvement; and type III, Types I+ II + bone involvement. The sample included four patients (28.57%) with PRS type I, six patients (42.85%) with PRS type II, and four patients (28.57%) with PRS type III.
RESULTS: Forty-seven procedures were performed. Free-fat grafts were used in all patients. Dermal fat grafts were used in all type II patients and one type III patient (25%). Bone grafts with temporoparietal fascia flaps were performed for the treatment of all type III patients. One type III patient (25%) underwent orthognathic surgery. All patients were improved in their overall facial appearance and there were no procedure-related complications.
CONCLUSION: Our proposed system for grading PRS severity can facilitate the choice of therapeutic approaches and with a combination of surgical techniques based on the severity of the disease partially satisfactory outcomes can be attained.
Keywords: Parry-Romberg syndrome; Progressive hemifacial atrophy; Surgical treatment; Grading system.
RESUMO
INTRODUÇÃO: Síndrome de Parry-Romberg (SPR) é caracterizada pela atrofia hemifacial progressiva que, muitas vezes, resulta em graves distúrbios estéticos e funcionais. Embora existam escalas de gravidade, nenhuma delas é completamente ideal para auxiliar na abordagem terapêutica destes pacientes. O objetivo deste estudo foi delinear as estratégias cirúrgicas para o tratamento da SPR baseado em um novo sistema de classificação de gravidade da doença.
MÉTODO: Trata-se de uma análise retrospectiva dos pacientes com SPR operados em 2005-2011. As abordagens cirúrgicas foram individualizadas de acordo com a escala de gravidade clínica baseada na evolução da doença: tipos I (envolvimento da epiderme, derme e tecido subcutâneo); II (tipo I + envolvimento muscular); e III (tipo I + II + envolvimento ósseo). Quatro (28,57%) pacientes com SPR tipo I, 6 (42,85%) tipo II e 4 (28,57%) tipo III foram incluídos.
RESULTADO: Um total de 47 procedimentos foi realizado. Gordura livre foi enxertada em todos os pacientes. Todos os pacientes do tipo II e 1 (25%) do tipo III foram submetidos a enxertos dermogordurosos. Enxertos ósseos com retalhos de fáscia têmporo-parietal foram aplicados no tratamento de todos os pacientes do tipo III. Um (25%) paciente do tipo III foi submetido à cirurgia ortognática. Houve melhora global na aparência facial em todos os pacientes, sem complicações relacionadas aos procedimentos.
CONCLUSÃO: O sistema de classificação de gravidade proposto para a SPR pode facilitar a decisão terapêutica e resultados parcialmente satisfatórios podem ser alcançados com a combinação de técnicas cirúrgicas de acordo com a gravidade da doença.
Palavras-chave: Síndrome de Parry-Romberg; Atrofia facial progressiva; Tratamento cirúrgico; Sistema de classificação.
The Parry-Romberg syndrome (PRS), also known as progressive hemifacial atrophy, is a rare craniofacial condition of unknown etiology. The disease is primarily characterized by progressive hemifacial atrophy, affecting the skin, subcutaneous tissues, muscles, nerves, cartilage, and, less frequently, the bones1,2. These alterations often lead to tridimensional asymmetry in the faces of these patients and are associated with severe functional and psychological disturbances1,2. As such, craniofacial surgeries, aimed at restoring facial symmetry, are a crucial part of the strategy leading to the full rehabilitation of these patients1,3. However, and in spite of the three previously reported grading systems for PRS severity,4,6 there is no consensus regarding the appropriate surgical procedures to be employed for each degree of severity1,3-6.
Thus, the aim of this study was to establish craniofacial surgical strategies for the treatment of PRS based on a new grading system for the severity of the Parry-Romberg syndrome.
METHODS
This is a retrospective study of all PRS patients surgically treated in the Sobrapar Hospital between 2005 and 2011. All aspects concerning surgical interventions were verified by analyzing the medical records, photographs, and clinical interviews. Patients with facial atrophy of known origin (trauma, burns, or craniofacial tumors) and patients with incomplete medical files and/or with incomplete postsurgical follow-up were excluded.
Fourteen patients with a diagnosis of PRS met the inclusion criteria. The average patient age was 19.4 years. Eleven patients (78.57%) were women and three (21.43%) were men. Eleven patients (78.57) had atrophy on the right side of the face, two (14.29%) on the left side, and one (7.14%) had bilateral facial atrophy.
Surgical interventions
The four surgical procedures (free-fat grafts,7,8 dermal fat grafts,9 cranial bone grafts with temporoparietal fascia flaps (TPFF),10,11 and orthognathic surgery12) used to correct the craniofacial defects of the PRS patients have been previously described.
Free-fat grafts:7,8 The collection, preparation, and injection of the free-fat grafts were based on the technique described by Coleman et al7. A 2-3mm diameter cannula connected to a 10 mL manual syringe (negative pressure) was used to collect the fat tissue preferentially from the lower abdomen or less frequently from the medial thigh. The aspirated tissue was centrifuged for 2 minutes at 2000 rpm. Following the removal of the supernatant, the fat was transferred to 1 mL syringes and injected into the affected facial regions. Multiple access points, multiple tunnels, and multiple layers were used to transfer small aliquots of fat, at different depths, to the hypoplastic facial regions. Approximately 0.1ml of fat was deposited with each cannula insertion.
Dermal fat grafts9: The longest horizontal axis of lower abdominal region was determined. This area was deepithelized (total thickness) with the aponeurosis of the rectus abdominis muscle serving as the lower limit for the dissection. The dermal fat graft was cut in the shape of a triangle and then introduced in the affected facial region; meticulous technique ensured minimal facial undermining.
Cranial bone grafts with TPFF10,11: The superficial temporal artery was carefully marked along its entire course. The initial incision of the scalp was performed in the most distal position in relation to the origin of the superficial temporal artery. The scalp was elevated in the subcutaneous plane towards the ear. Following the complete elevation of the scalp skin graft, the graft's axial pedicle was exposed. The thin superficial temporoparietal fascia was then elevated in its entirety, followed by dissection in the subgaleal plane. The periosteal flap was delineated and elevated to expose the frontal bone and the orbital cone. A craniotomy was performed in the parietal area. The parietal bone, in its entire thickness, was divided. One subciliary incision with zygomaticomaxillary exposure allowed the correct fixation of segments of the bone flap to the recipient hypoplastic region with 1.5mm screws. The external bone flap was returned to the donor parietal region. The TPFF was then rotated towards the region of the facial deformity to cover the bone flap.
Scale for grading clinical severity
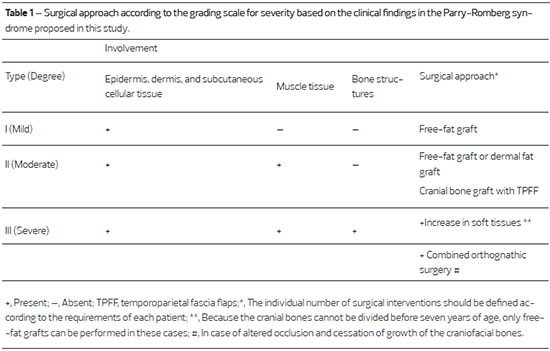
All surgical approaches were individually considered according to the grading scale for the severity of the disease developed by our group. The distinction between the three degrees of severity was based on photographs, and on clinical, radiographic, and tomographic examinations, The three grades were: mild (type I), with epidermal, dermal, and subcutaneous tissue involvement; moderate (type II), with epidermal, dermal, and subcutaneous tissue involvement, as well as muscle involvement; and severe (type III), with epidermal, dermal, and subcutaneous tissue involvement, as well as muscle and bone involvement (Table 1). Based on this scale, four (28.57%) patients were classified as type I, six (42.85%) patients as type II, and four (28.57%) patients as type III.
Evaluation of craniofacial surgical results
All surgical results were evaluated by the same plastic surgeon who had no previous contact with the patients. Pre-operative frontal, oblique, and profile facial photographs, taken days or weeks before the first surgical procedure, were compared to post-surgery photographs taken 12-14 months after the last surgical procedure. Photographs were classified according to a scale for grading the degree of improvement of the facial symmetry previously used in PRS8: (a) satisfactory result, symmetrical face with no need for additional interventions; (b) partially satisfactory result, overall improvement of the facial appearance but facial asymmetry can still be observed after careful examination; and (c) unsatisfactory result, lack of evident improvement in facial symmetry after the surgical interventions.
RESULTS
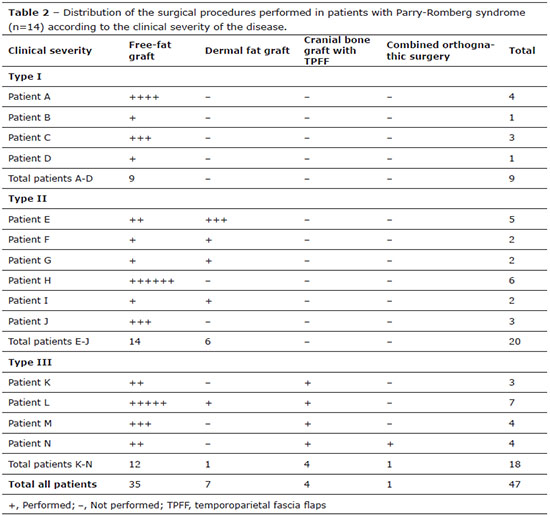
The indications for surgical procedures were asymmetry/facial malformation (100%), hypoplasia of the soft tissues (71.43%), and hypoplasia of the soft tissues and bones (28.57%). In total, 47 craniofacial surgical interventions were performed based on the clinical severity of each patient's disease and also on the basis of the level of soft tissue alterations detected by the pre-surgical evaluations. The number of surgeries performed in each patient was variable (1-6 procedures/patient). The average number of interventions per patient was 2.5 procedures/patient in type I PRS patients, 3.33 procedures/patient in type II patients, and 4.5 procedures/patient in type III patients. All patients received free-fat grafts at some point during the treatment period (type I: 1-4 free-fat grafts/patient; type II: 1-6 free-fat grafts/patient; and type III: 2-5 free-fat grafts/patient). Five (35.71%) patients received dermal fat grafts (type I: none; type II: 1-3 dermal fat grafts/patient; and type III: 1 dermal fat graft/patient). Four patients (28.57%) had cranial bone grafts with TPFF (types I and II: none; and type III: 1 cranial bone graft/patient) and one patient (7.14%) underwent orthognathic surgery (types I and II: none; and type III: 0.25 TPFF/patient) (Table 2).
Utilizing the different combinations of surgeries we attained an overall improvement in the facial appearance (partially satisfactory result) in all patients (Figures 1-5). In this series, there was no satisfactory or unsatisfactory result according to the scale introduced by Xie et al.8 A variable degree of fat absorption was detected following free-fat grafting. There was no infection or necrosis in any of the dermal fat grafts or cranial bone grafts with TPFF. In addition, there were no complications in the donor areas.
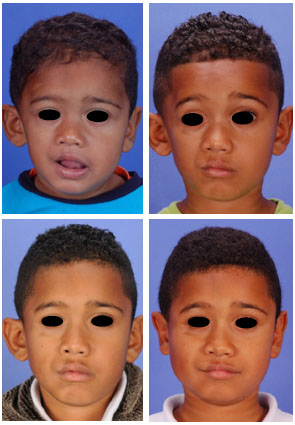
Figure 1 - (Above, left) Pre-surgical frontal photographs of a three-year-old type I Parry-Romberg syndrome patient showing early manifestations of the disease (Table 2, patient D). (Above, right) Frontal photograph of the same patient, two years later, showing the slow progression of the disease in this case. (Below, left) At seven years of age, the patient underwent hemifacial free-fat inclusion and otoplasty for correction of his prominent ears. (Below, right) Two years after the surgery, the patient had a satisfactory esthetic result, with maintenance of the volume in the right hemiface and without any signs of recurrent ear deformity.
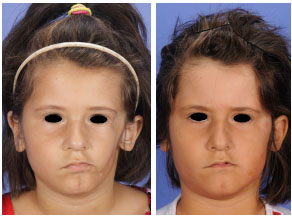
Figure 2 - (Left) Pre-surgical frontal photographs of a 6-year-old type II Parry-Romberg syndrome patient (Table 2, Patient J). (Right) Post-surgical frontal photograph of the same patient, three years after initial hemifacial (left) free-fat inclusion surgery.

Figure 3 - (Left) Pre-surgical oblique photographs of the same patient as in Figure 2. A hyperchromic stain in the middle third of the face can be observed. (Right) Post free-fat inclusion surgery oblique photographs of the same patient showing a significant improvement in the facial atrophy and hyperchromic stain that covered the entire middle third of the child's face.
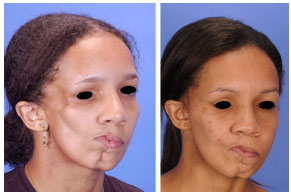
Figure 4 - (Left) Pre-surgical frontal photograph of a type III Parry-Romberg syndrome patient (Table 2, patient N). (Right) Post-surgical frontal photograph of the same patient after the following series of surgeries: free-fat graft, parietal bone grafts with rotation of the superficial temporal fascia flaps over the bone grafts, and combined orthognathic surgery and free-fat graft.
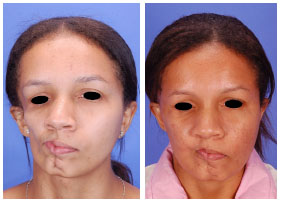
Figure 5 - (Left) Pre-surgical oblique photograph of the same patient as in Figure 4. The severe facial atrophy can be observed. (Right) Post-surgical oblique photograph of the same patient showing significant improvement of the facial atrophy.
DISCUSSION
PRS is a craniofacial deformity of unknown etiology, with a higher prevalence in women. It affects the face unilaterally in 95% of cases and usually has its onset during the first or second decades of life, with an active phase spanning 2-10 years before clinical stabilization1,2.
Two Mexican4,5 and one Chinese6 classification systems have previously been used to grade the clinical severity of the disease. The grading system by Iñigo et al4. is based on the involvement of tissues in the region of the trigeminal nerve (dermatomes), whereas the classifications by Guerrerosantos et al.5 and Hu et al.6 are based uniquely on tissue involvement. In this study, we propose a severity scale related to the clinical progression of the disease, associated with anatomical involvement, independent of the affected dermatome.
The onset of PRS appears to be associated with atrophy of the epidermis, dermis, and subcutaneous tissue, with progressive involvement of the facial muscles followed by bony involvement1,2. Thus far, there have been no reports in the literature of bony involvement without disease in the surrounding soft tissues. Actually, a close relation between bone involvement and the severity of facial atrophy has been reported. Additionally, the restriction imposed by the deformed soft tissues leads to compromised bone growth13. Therefore, type III disease represents the progression of types I and II disease, and type II is the result of the progression of type I disease. These degrees of disease severity reflect the progression of the disease itself, which suggests that this classification is both logical and functional. In addition, this classification can be used to guide the therapeutic approach to patients with PRS.
It should be emphasized that this grading system was based solely on the analysis of 14 patients with PRS and is supported by other studies4-6 with limited numbers of patients. As such, multicenter studies, including a larger number of patients, should be done so that the clinical progression of PRS can be better characterized, and potentially corroborate our findings.
Because PRS is usually associated with tridimensional craniofacial deformities, the complete restoration of facial symmetry is often difficult to achieve3-6. Furthermore, there is no consensus regarding the ideal surgical treatment for the wide spectrum of soft tissue and bone hypoplasia found in PRS, though several therapeutic options (free-fat grafts, dermal fat grafts, cartilage and bone grafts, muscle flaps, and alloplastic implants) have been described with the aim of increasing the facial volume lost to progressive atrophy1,3-6,14-19.
The clinical phase, whether active or stable, is highly relevant in determining the optimal surgical treatment of PRS. There is no consensus in the literature regarding the ideal time for correcting the patient's deformities8,18. Traditionally, surgical procedures have only been performed after the stabilization of facial atrophy.6,8,18 However, it should be noted that the time required for stabilization of the disease is variable and unpredictable14,16, and children with PRS can develop psychosocial disturbances while awaiting surgery14. This is particularly true with regard to peer interactions in school for example, and may directly impact the patient's quality of life. In light of these findings, our group and others14,16 have supported the view that craniofacial surgical interventions should be performed earlier, especially in those children with craniofacial deformities that, if left untreated, might lead to psychosocial impairments and learning disabilities. Early intervention may potentially improve or maintain physical capacities, levels of independence, and social relations at a critical time in the development of the patient14.
In craniofacial surgery clinics, the protocols for treatment of craniofacial hypoplasias are guided primarily by facial growth and the functional needs of the patients20. In addition, a successful therapeutic approach requires individualized treatments employing reconstructive procedures at the soft tissue and bone level, performed at the appropriate time.20 Taking these principles into consideration, the current therapeutic approach for PRS consists, in general, of either only one procedure or combined surgical procedures.1,2 Our group and others4-6,14,15,17 have treated each patient according to the degree of tissue atrophy4-6,14,16 and also according to the age of the patient14,15.
Recently, a therapeutic algorithm addressing hypoplasia of the soft tissues in PRS has been described. However, bone defects in these patients were not considered in the grading system15. In patients with type III disease an increase in soft tissue alone is not sufficient18. Therefore, we have favored a therapeutic strategy that combines the reconstruction of both soft tissue and bone: free-fat grafts for those patients with type I PRS, free-fat grafts or dermal fat grafts for type II PRS patients, and bone grafts with TPFF for type III PRS patients (Figure 6).
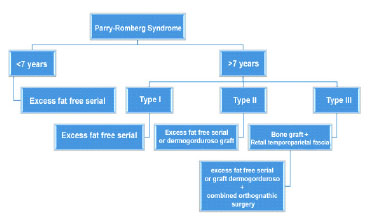
Figure 6. Therapeutic algorithm for the surgical treatment of hypoplasia of the soft tissues and craniofacial bones in patients with Parry-Romberg syndrome based on clinical severity.
In our hospital, as well as at the UCLA Craniofacial Clinic14-16,20, the serial free-fat graft has been the first-choice method for the reconstruction of soft tissues in several craniofacial abnormalities, including PRS. Along with other authors8,14,15,20, we prefer the free-fat graft instead of the injection of synthetic materials that more commonly lead to infection, seroma, exposure, and migration14,15,20. With regard to free-fat grafts, it should be noted that adipose tissue is among the tissues with highest angiogenic capacity21. A series of studies have shown that free-fat grafts are associated with local neo-angiogenesis, which indicates that this intervention contributes to an improved vascularization of the receiving area. In PRS, compromised vascularization in the affected area may be the cause of soft tissue atrophy, and consequently, atrophy of the underlying bony structure18. Therefore, we believe that free-fat grafting can alter the prognosis of patients with type I PRS, as it may slow disease progression through improvement of local vascularization. Other investigators have also reported that the vascularized free flap can potentially act as a barrier against PRS progression18. Future studies should focus on the angiogenesis associated with free-fat grafts, as well as on their impact on PRS progression, perhaps employing Doppler scans to measure disease progression.
The variability in the survival rate of free-fat grafts has been reported as one of the main concerns with regard to this therapeutic approach15,24. We, along with others8, have shown subjectively that patients with PRS absorb a certain degree of the grafted free-fat. However, no specific research on the level of free-fat absorption, or on the factors that may decrease absorption, have been done. Any information regarding the existence of fat absorption should be interpreted cautiously. One published study16 investigated the rate of retention of the free-fat graft in the post-surgical period (1 year) and claimed that the "stickiness" of the free-fat graft is lower in PRS patients than in healthy patients without PRS. This diminished incorporation of the free-fat graft can be a consequence of both the poor vascularization at the receptor site, as well as other intrinsic characteristics of the PRS-affected region16.
In addition, the age of the PRS patient can influence the survival of the free-fat grafts16. Laboratory studies26,27 have shown that free-fat grafts are more effective in younger patients. Besides age, other factors have been reported to influence the absorption rate of free-fat grafts26-28 including sedimentation rate, the nutritional status of the patient, and body mass index. However, there is no consensus on the actual impact of these variables on the absorption of free-fat grafts22-28. Hence, future research should focus on defining the roles of these different factors on the rate of incorporation of free-fat grafts. Meanwhile, the use of free-fat grafts, in particular in PRS patients, should be based on objective data, such as the volumetric tridimensional photogrammetric analysis of the faces of these patients. This type of analysis has demonstrated the improvement in symmetry and in the facial volume of patients one year after the free-fat injections, when compared with facial symmetry and facial volume prior to the intervention16. Furthermore, the impact of surgical interventions in the psychosocial context and quality of life of these patients should also be considered when choosing a therapeutic approach14.
Assuming that some degree of fat absorption will occur in the post-surgical period,8,15,16 we and others8,14,16,27 prefer to over-correct the facial defect when transferring autogenic tissue. Xie et al8. have over-corrected by 20-30% of the total injected volume, as it has been reported that about 70-80% of the free-fat can survive following graft transplantation in PRS patients8. Along with others, we have used grafts with approximately 10% more volume than that required to achieve symmetry in the patient's face with the aim of attaining a higher survival rate of the fat and decreasing the degree of fat necrosis and the formation of palpable nodules27. In addition, the free-fat graft procedure can be performed several times without worsening the disease, based on the individual patient's requirements during the follow-up period8,14-16.
Decreased blood circulation can be demonstrated in the atrophic facial tissues, specifically in type III patients. This may decrease the viability of the graft following surgery6. In these cases, we prefer TPFF, as this approach provides additional blood flow to the bone graft, as well as to fat grafts that may be placed in the future. In this series we did not observe clinically relevant reabsorption of any of the bone grafts associated with TPFF, which contrasts with the findings of other studies14 that have used layering of bone grafts without additional blood flow. Our findings are in line with a study18 that previously reported improved results by combining rib bone grafts with free dermal fascia flaps of the lateral intercostal artery perforator.
We prefer to use the TPFF procedure as this flap has several advantages such as a flexible outline, high vascularization with a wide pedicle rotation arch, anatomic proximity to structures in the face, and minimal morbidity of the donor site, among others11,29, that facilitate and contribute to the symmetrical and harmonious reconstruction of the face. This procedure also assures adequate blood flow to the associated cranial graft without the need for microsurgical procedures that require additional skills15.
There is no consensus in the literature concerning the age at which PRS patients should undergo surgical therapy. Many authors contend that correction of hypoplasia of the soft tissues should be performed only after treatment of the bony defects14,16. Meanwhile, we agree with others who have treated patients as early as possible, independent of the clinical severity of the disease16. Satisfaction associated with the results of surgery has been higher for the youngest PRS patients, even if more surgeries were required during the follow-up period14. It should be emphasized that before reaching seven years of age, patients have insufficient donor regions and the parietal bone is not thick enough to be divided30. Therefore, patients younger than seven years old are limited to serial free-fat grafting.
The maturity of the craniofacial bones is also relevant in the decision-making process using a different therapeutic algorithm15, since, as previously mentioned, only the reconstruction of soft tissue defects has been graded15. As the bones of patients with type III disease become involved, malocclusion can occur as the craniofacial bones stop growing. These patients may have skeletal open-bite malocclusion as a consequence of progressive unilateral atrophy of the jaw. Therefore, coordinated planning of these procedures by the plastic surgeon and the orthodontic surgeon is paramount. According to our experience and that of others6,14-16, such patients can be considered candidates for combined orthognathic surgeries with or without osteodistraction, aimed at decreasing facial asymmetry, and may be followed by augmentation of the soft tissues with the use of serial free-fat grafts.
Our study, similar to previous studies8, evaluated the results of craniofacial surgical procedures subjectively with the use of a previously described scale in PRS patients8. All patients had an overall improvement in facial appearance. However, a carefully conducted evaluation showed residual facial asymmetry and additional interventions were likely required, as showed in Figures 4 and 5. In addition, and despite the partially satisfactory results achieved, the analysis method8 used has limitations, as it is based on a static characterization of a dynamic disease and therapeutic interventions that change over time (i.e., the absorption of grafts or flaps). Follow-up continues for all of the PRS patients reported here and new surgical approaches will be adopted in the future depending on the individual needs of each patient. Future studies using objective analytic methods, such as photogrammetric volumetric quantification or tomography, are expected to help elucidate the nature of PRS and the optimal therapy for this condition.
CONCLUSION
In this retrospective study, the therapeutic approach to patients with PRS was guided by the graded severity of the craniofacial deformation. The scale for grading severity proposed here describes the clinical stages of the disease and contributes to the decision-making process when considering the different therapeutic approaches in PRS patients.
REFERENCES
1. Hunt JA, Hobar PC. Common craniofacial anomalies: conditions of craniofacial atrophy/hypoplasia and neoplasia. Plast Reconstr Surg. 2003;111(4):1497-508.
2. El-Kehdy J, Abbas O, Rubeiz N. A review of Parry-Romberg syndrome. J Am Acad Dermatol. 2012;67(4):769-84.
3. Wójcicki P, Zachara M. Surgical treatment of patients with Parry-Romberg syndrome. Ann Plast Surg. 2011;66(3):267-72.
4. Iñigo F, Rojo P, Ysunza A. Aesthetic treatment of Romberg's disease: experience with 35 cases. Br J Plast Surg. 1993;46(3):194-200.
5. Guerrerosantos J, Guerrerosantos F, Orozco J. Classification and treatment of facial tissue atrophy in Parry-Romberg disease. Aesthetic Plast Surg. 2007;31(5):424-34.
6. Hu J, Yin L, Tang X, Gui L, Zhang Z. Combined skeletal and soft tissue reconstruction for severe Parry-Romberg syndrome. J Craniofac Surg. 2011;22(3):937-41.
7. Pu LL, Coleman SR, Cui X, Ferguson RE Jr, Vasconez HC. Autologous fat grafts harvested and refined by the Coleman technique: a comparative study. Plast Reconstr Surg. 2008;122(3):932-7.
8. Xie Y, Li Q, Zheng D, Lei H, Pu LL. Correction of hemifacial atrophy with autologous fat transplantation. Ann Plast Surg. 2007;59(6):645-53.
9. Raposo do Amaral CE, Cetrulo CL Jr, Pereira CL, Guidi Mde C, Raposo do Amaral CM. Augmentation gluteoplasty with dermal-fat autografting from the lower abdomen. Aesthet Surg J. 2006;26(3):290-6.
10. Brent B, Byrd HS. Secondary ear reconstruction with cartilage grafts covered by axial, random, and free flaps of temporoparietal fascia. Plast Reconstr Surg. 1983;72(2):141-52.
11. Raposo-do-Amaral CE, Raposo-do-Amaral CA, Guidi M, Buzzo C. The role of temporoparietal fascia flap in craniofacial skeleton and secondary ear reconstruction. Rev Bras Cir Craniomaxilofac. 2010;13(1):1-6.
12. Thaller SR, Bradley JP, Garri JI. Craniofacial Surgery. New York: Informa Healthcare USA; 2007.
13. Moore MH, Wong KS, Proudman TW, David DJ. Progressive hemifacial atrophy (Romberg's disease): skeletal involvement and treatment. Br J Plast Surg. 1993;46(1):39-44.
14. Slack GC, Tabit CJ, Allam KA, Kawamoto HK, Bradley JP. Parry-Romberg reconstruction: optimal timing for hard and soft tissue procedures. J Craniofac Surg. 2012;23(7 Suppl 1):1969-73.
15. Tanna N, Broer PN, Roostaeian J, Bradley JP, Levine JP, Saadeh PB. Soft tissue correction of craniofacial microsomia and progressive hemifacial atrophy. J Craniofac Surg. 2012;23(7 Suppl 1):2024-7.
16. Slack GC, Tabit CJ, Allam KA, Kawamoto HK, Bradley JP. Parry-Romberg reconstruction: beneficial results despite poorer fat take. Ann Plast Surg. 2014;73(3):307-10.
17. Yu-Feng L, Lai G, Zhi-Yong Z. Combined treatments of facial contour deformities resulting from Parry-Romberg syndrome. J Reconstr Microsurg. 2008;24(5):333-42.
18. Myung Y, Lee YH, Chang H. Surgical correction of progressive hemifacial atrophy with onlay bone graft combined with soft tissue augmentation. J Craniofac Surg. 2012;23(6):1841-4.
19. Cardoso LA, Carvalho PM, Kohatsu EM, Cardoso KT, Alves KV, Souza TL. Uma nova opção cirúrgica para a Síndrome de Romberg. Acta Medica Misericordiae. 2000;3(1):32-5.
20. Lim AA, Fan K, Allam KA, Wan D, Tabit C, Liao E, et al. Autologous fat transplantation in the craniofacial patient: the UCLA experience. J Craniofac Surg. 2012;23(4):1061-6.
21. Lemoine AY, Ledoux S, Larger E. Adipose tissue angiogenesis in obesity. Thromb Haemost. 2013;110(4):661-9.
22. Sultan SM, Barr JS, Butala P, Davidson EH, Weinstein AL, Knobel D, et al. Fat grafting accelerates revascularisation and decreases fibrosis following thermal injury. J Plast Reconstr Aesthet Surg. 2012;65(2):219-27.
23. Hamed S, Ben-Nun O, Egozi D, Keren A, Malyarova N, Kruchevsky D, et al. Treating fat grafts with human endothelial progenitor cells promotes their vascularization and improves their survival in diabetes mellitus. Plast Reconstr Surg. 2012;130(4):801-11.
24. Tabit CJ, Slack GC, Fan K, Wan DC, Bradley JP. Fat grafting versus adipose-derived stem cell therapy: distinguishing indications, techniques, and outcomes. Aesthetic Plast Surg. 2012;36(3):704-13.
25. Yuan Y, Gao J, Liu L, Lu F. Role of adipose-derived stem cells in enhancing angiogenesis early after aspirated fattransplantation: induction or differentiation? Cell Biol Int. 2013;37(6):547-50.
26. Zhu M, Kohan E, Bradley J, Hedrick M, Benhaim P, Zuk P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regen Med. 2009;3(4):290-301.
27. Kanchwala SK, Bucky LP. Invited discussion: correction of hemifacial atrophy with autologous fat transplantation. Ann Plast Surg. 2007;59(6):654.
28. Mojallal A, Lequeux C, Shipkov C, Duclos A, Braye F, Rohrich R, et al. Influence of age and body mass index on the yield and proliferation capacity of adipose-derived stem cells. Aesthetic Plast Surg. 2011;35(6):1097-105.
29. Collar RM, Zopf D, Brown D, Fung K, Kim J. The versatility of the temporoparietal fascia flap in head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2012;65(2):141-8.
30. Tessier P. Autogenous bone grafts taken from the calvarium for facial and cranial applications. Clin Plast Surg. 1982;9(4):531-8.
1 - MD, PhD; Full member of the Brazilian Society of Plastic Surgery (Sociedade Brasileira de Cirurgia Plástica, SBCP) and of the Brazilian Association of Craniomaxillofacial Surgery (Associação Brasileira de Cirurgia Crânio-Maxilo-Facial, ABCCMF); PhD degree from the program of the Surgical Center of the University of São Paulo (USP); vice-president of the Institute of Craniofacial Plastic Surgery of the Hospital of Sobrapar, Campinas, SP, Brazil
2 - MD; Aspiring member in training of the SBCP; first year resident of plastic surgery at the Hospital of Sobrapar, Campinas, SP, Brazil
3 - MD, MSc; Full member of the SBCP and of the ABCCMF; Master degree in Surgery from the State University of Campinas (UNICAMP); Full Professor of the Plastic Surgery Clinic "Prof. Dr. Cassio M. Raposo do Amaral" of the Institute of Craniofacial Plastic Surgery of the Hospital of Sobrapar, Campinas, SP, Brazil
4 - Full member of the SBCP; Preceptor of the Residents of the Plastic Surgery Clinic "Prof. Dr. Cassio M. Raposo do Amaral" of the Institute of Craniofacial Plastic Surgery of the Hospital of Sobrapar, Campinas, SP, Brazil
Institution: Study performed at the Institute of Craniofacial Plastic Surgery of the Hospital of SOBRAPAR, Campinas, SP, Brazil.
Corresponding author:
Cassio Eduardo Raposo-do-Amaral
Hospital de Crânio e Face SOBRAPAR
Av. Adolpho Lutz, 100 - Cidade Universitária
Campinas, SP, Brasil - CEP 13083-880; Caixa-postal 6028
E-mail: cassioraposo@hotmail.com
Article received: August 19, 2013
Article accepted: 30 October 30, 2013


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter